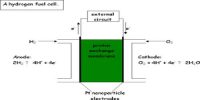
A fuel cell is a device that generates electricity by a chemical reaction. A proton-conducting polymer membrane (the electrolyte) separates both the anode and cathode sides as shown below.
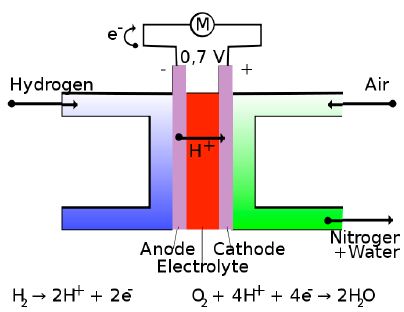
A fuel cell is essentially a battery, but differs by operating with a continuous supply of energetic reactants, or fuel. Every fuel cell has two electrodes, one positive and one negative, called, respectively, the anode and cathode.
For a hydrogen-oxygen fuel cell, the electrode reactions are as follows:
Anode: H2 (g) → 2H+ (aq) + 2e–
Cathode: O2 (g) + 4H+ (aq) + 4e– → 2H2O (I)
Different types of fuel cells: Alkali fuel cells, Molten Carbonate fuel cells (MCFC), Phosphoric Acid fuel cells (PAFC), Proton Exchange Membrane (PEM) fuel cells, Solid Oxide fuel cells (SOFC) etc.