The alignment of the dipole moments of the permanent or induced dipoles in the direction of applied electric field is called polarization or electric polarization.
A nonpolar molecule is one in which the centre of gravity of the positive charges (protons) coincide with the centre of gravity of the negative charges (electrons). Example: O2, N2, H2. The nonpolar molecules do not have a permanent dipole moment.
If a non polar dielectric is placed in an electric field, the centre of charges get displaced. The molecules are then said to be polarized and are called induced dipoles. They acquire induced dipole moment p in the direction of electric field (Figure).
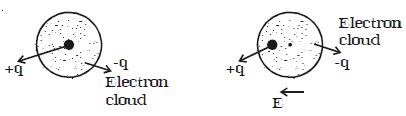
Fig: Induced dipole