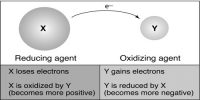
The word redox is formed by the combination of two words which are reduction and oxidation. That means, red from reduction combines with ox from oxidation producing redox. So, the reaction in which reduction and oxidation both take place simultaneously is known as redox reaction.
The processes which involve the gaining of electron or electrons is called reductions and the process which involves the releasing of electron or electrons is called oxidation.
For example,
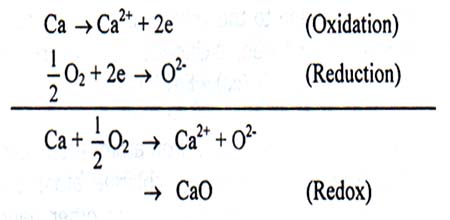
Here Ca undergoes oxidation by releasing two electrons and oxygen undergoes reduction by gaining two electrons. Both oxidation and reduction take place in the same oxidation-reduction reaction. So, this is a redox reaction.