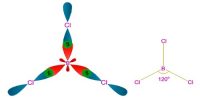
The process of formation of 4 equivalent orbitals from hybridization or mixing up of one S– and three P orbitals is known as sp3 hybridization. sp³ hybrid orbitals and properties of sigma bonds.
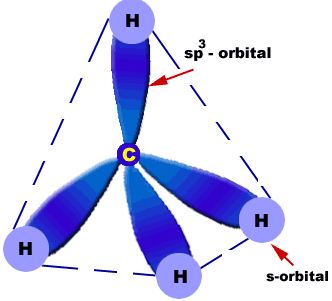
Characteristics:
- sp3 has 25% s and 75% p character
- the 4 sp3 hybrids point towards the corners of a tetrahedron at 109.5o to each other
- each sp3 hybrid is involved in a s bond.