A mole is defined as the amount of substance which contains different number of particles like; atoms, molecules, ions etc., as there are atoms in exactly 12.000 gm of Carbon-12.
One mole of Carbon-12 atom has a mass of exactly 12.000 grams and contains (6.02 * 10^23) atoms.
A mole is just a number like a Dozen. A dozen equals to 12 eggs, a gross of pencil equals to 144 pencil, similarly mole is equal to (6.02 * 10^23) atoms, here is Avogadro Constant. Mole is also known as chemist dozen.
The value of (6.02 * 10^23) is known as Avogadro Constant, after the Italian scientist who first recognized the importance of the mass/number relationship.
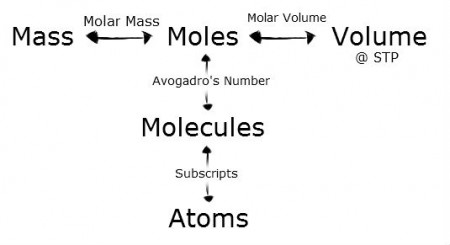 Main concepts:
Main concepts:
- 6.02 x 1023 of anything.
- The formula mass in grams of a substance contains one mole of particles.
- Na = Avogadro’s Number = 6.02 x 1023 Spreading a “mole” of marbles over the entire surface of the earth would produce a layer about three miles thick.

Mole Examples: (Avogadro’s Revenge)
- Astronomers estimate that there is a mole (6.02 x 1023) of stars in the universe.
- One mole of high school chemistry textbooks would cover the USA to a depth of about 320 km (200 miles).
- Water flows over Niagara Falls at about 650,000 kL (172,500,000 gallons) per minute. At this rate it would take 134,000 years for one mole of water drops (6.02 x 1023 drops) to flow over Niagara Falls.
- Avogadro’s number (6.02 x 1023) is the approximate number of milliliters of water in the Pacific Ocean (7 x 108 km3 or 7 x 1023 mL).











