A new study has discovered the mechanism underlying the inflammation that is a characteristic of the prevalent skin illness. Eczema is characterized by dry, itchy, and red skin (atopic dermatitis). It’s a common, non-contagious skin ailment. If you have asthma or allergies, you are at a higher risk.
Atopic dermatitis, a common skin ailment that affects both children and adults, is commonly thought of as an inflammatory disease caused by a breakdown in the skin’s barrier function. A new study has identified a cascade of inflammatory signaling that occurs before to the formation of skin ulcers, shedding insight on the condition’s early stages and proposing potential novel molecular targets for therapeutic intervention.
The study, which was published in the journal Science Translational Medicine, was the result of a cross-school and cross-institutional collaboration between researchers at the University of Pennsylvania’s School of Dental Medicine and Perelman School of Medicine, Oak Ridge National Laboratory, and the University of Tennessee.
“You have researchers in the dental school noticing a skin condition and broadening their work to the medical school and experts on computational systems biology,” says Dana Graves, a co-corresponding author on the paper and a professor and vice dean for research and scholarship at Penn Dental Medicine. “Without this interdisciplinary teamwork, that initial finding would not have gone anywhere.”
John Seykora, a co-corresponding author and dermatology professor at Penn Medicine, agrees. “This demonstrates one of the advantages of being part of a university with expertise from other sectors,” he explains. “Our dentistry school colleagues created a mouse with a specific skin phenotype, and the question was, what was this, and did it mirror any condition we would be familiar with? And, in the end, it did, revealing some unique insights into a very prevalent human skin problem.”
A significant result from our research was that fibroblasts can affect immune cells. Our findings, together with recent findings in other inflammatory diseases, suggest that fibroblasts are crucial regulators of white blood cells.
Kang Ko
The work began in Graves’ lab, with Kang Ko, then a student in the Doctor of Science in Dentistry program and now a Penn Dental Medicine assistant professor, leading an exploration of the role of inflammatory signaling in bone fracture healing in diabetes. A focus was on nuclear factor kappa-B (NF-kB), a master regulator of inflammatory responses. As part of that work, Ko, Graves, and colleagues developed a mouse model lacking an activator of NF-kB signaling, IKKB.
As the mice grew older, the researchers discovered that they acquired skin sores. “We were intrigued because these ulcerations appeared to be an inflammatory occurrence, yet we had effectively turned down the activity of NF-kB, which should minimize inflammation,” Graves says. “There was a paradox here.”
They sought expertise in skin diseases to better understand what was causing this response. “Obviously, as someone who studies bone and oral biology, the issue of dermatology seemed a little different,” Ko adds.
Ko and Graves knew of Seykora from a talk he had given about the Cutaneous Phenomics and Transcriptomic core which he directs, part of Penn’s Skin Biology and Diseases Research-based Center housed in Dermatology. When Seykora and colleagues examined the mice, they noted several features quite similar to atopic dermatitis, “albeit the mouse version,” he says.
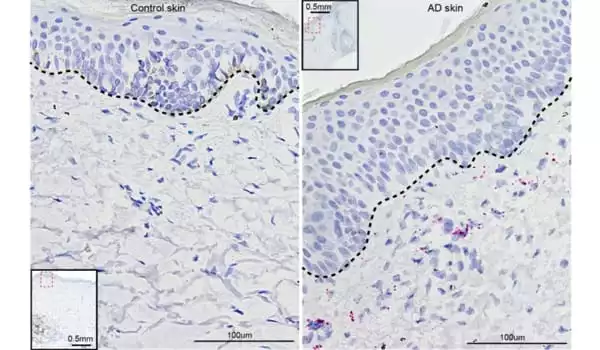
They observed skin thickening and infiltration of specific types of white blood cells, which are likewise reported in human atopic dermatitis. To learn more about how the loss of IKKB was causing these effects, the researchers used single-celled RNA analysis, a technique that determines which RNA transcripts are present in individual cells.
They then collaborated with computational systems biology experts Daniel Jacobson of Oak Ridge National Laboratory and his student Jean Merlet at the University of Tennessee, who had developed a new explainable-AI-based method for analyzing single-cell RNA sequencing data with other students in Jacobson’s lab. Together, the team discovered that the cell type that appeared responsible for these effects was fibroblasts, a major component of the skin’s dermis layer and typically thought to support the structural integrity of skin.
“We are excited that these new methods that we have developed for single-cell RNA-seq analysis have led the discovery of such exciting biology implicating the important role that NF-kB plays in fibroblasts in the context of atopic dermatitis” says Jacobson. “We believe that this type of transdisciplinary collaboration is crucial for solving the challenging problems that we face in complex biological systems.”
Though NF-kB normally promotes inflammation, in this case, lower NF-kB activity paradoxically led to immune cell recruitment and accompanying inflammation. Data from the team’s single-cell RNA analysis revealed that a protein transcription factor termed CEBPB was highly activated in the fibroblasts missing IKKB. CCL11 was also highly active in these fibroblasts. CCL11, also known as eotaxin, is a signaling molecule that causes inflammation by attracting a kind of white blood cell known as eosinophils to its site. “We worked out the mechanism in the mouse, then demonstrated that much of it applied in human tissue as well,” Sekora explains.
When the researchers matched what they saw in the mouse cells to skin samples from humans with atopic dermatitis, they discovered similar patterns; CCL11 and CEBPB were both identified at higher levels in the affected skin than in the unaffected skin. They also did parallel tests in human skin fibroblasts with an IKKB inhibitor and discovered that CCL11 levels increased substantially.
Returning to the mouse model, the researchers discovered that adding a monoclonal antibody against CCL11 reduced the inflammatory response seen in animals lacking IKKB, implying that this route could be one to target to reduce atopic dermatitis-associated inflammation.
The findings not only lead to a new route to therapy development for atopic dermatitis, but they also highlight a growing understanding that fibroblasts play crucial roles in immunological processes in the skin, according to the researchers.
“A significant result from our research was that fibroblasts can affect immune cells,” adds Ko. “Our findings, together with recent findings in other inflammatory diseases, suggest that fibroblasts are crucial regulators of white blood cells.”
Atopic dermatitis is a disorder that usually appears in childhood, commonly in conjunction with asthma. Indeed, the researchers detected signaling anomalies in mice at a period equivalent to the animals’ “childhood.” The findings of the study show that fibroblasts may be active during this phase in establishing adequate immunological signals in the skin.
“We’ve thought of NF-kB as a factor that promotes inflammation, but it’s possible that its activation throughout development is vital for preserving homeostasis,” Graves adds. Graves, Ko, Seykora, and Jacobson are continuing their work in order to further investigate NF-kB signaling in fibroblasts.
“Our goal is to refine and extend these investigations,” Seykora explains. “If all of this holds true, we might be able to pique people’s curiosity in targeting some of these pathways with new medicines.”