Storing and retrieving hydrogen in a safe, easy, and cost-effective manner is a continual challenge, but some promising approaches have been discovered or are being researched. Because hydrogen has a poor energy density per unit volume, it is difficult to store.
Researchers at Japan’s RIKEN Centre for Emergent Matter Science (CEMS) have identified a molecule that stores ammonia through a chemical reaction, potentially providing a safer and easier approach to storing this crucial chemical. This discovery, which was published in the Journal of the American Chemical Society on July 10, allows for the safe and convenient storage of not only ammonia, but also the vital hydrogen it carries. This discovery should pave the road for a decarbonized civilization with a viable hydrogen economy.
To transition from carbon-based to hydrogen-based energy, humanity requires a safe mechanism to store and transport hydrogen, which is highly flammable on its own. One method is to store it as a component of another molecule and retrieve it when needed. Ammonia, chemically abbreviated as NH3, is an excellent hydrogen carrier since each molecule contains three hydrogen atoms, with about 20% of ammonia being hydrogen by weight.
There are various applications for the novel storing technology. In the short term, we hope that this simple and efficient method can be a part of the solution for achieving a decarbonized society through the use of ammonia as a carbon-free hydrogen carrier.
Yoshihiro Ito
However, because ammonia is a very corrosive gas, it is difficult to store and use. Currently, ammonia is commonly stored by liquefying it in pressure-resistant containers at temperatures considerably below freezing. Porous compounds can also store ammonia at normal temperature and pressure, although the storage capacity is limited and the ammonia is not always easily retrievable. The latest research describes the development of a perovskite, a material with a peculiar repeating crystal structure that can easily store ammonia and also allows for easy and full retrieval at low temperatures.
Masuki Kawamoto’s team at RIKEN CEMS focused on the perovskite ethylammonium lead iodide (EAPbI3), also known chemically as CH3CH2NH3PbI3. They discovered that at ordinary temperature and pressure, its one-dimensional columnar structure conducts a chemical reaction with ammonia, dynamically transforming into a two-dimensional layered structure known as lead iodide hydroxide, or Pb(OH)I. As a result of this process, ammonia is chemically converted and stored inside the layered structure. Thus, EAPbI3 can securely store corrosive ammonia gas as a nitrogen compound in pressurised containers at -33°C (-27.4°F) in a considerably cheaper procedure than liquification. Even more significantly, retrieving the stored ammonia is a simple operation.
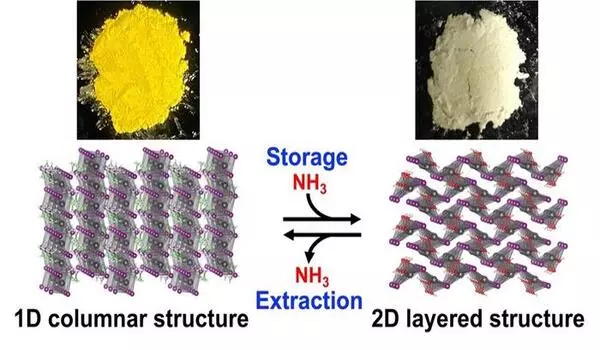
“To our surprise, ammonia stored in ethylammonium lead iodide could be easily extracted by heating it gently,” says Kawamoto. The stored nitrogen compound undergoes a reverse reaction at 50°C (122°F) under vacuum and returns to ammonia. This temperature is much lower than the 150°C (302°F) or more that is needed to extract ammonia from porous compounds, making EAPbI3 an excellent medium for handling corrosive gases in a simple and cost-effective process. Additionally, after returning to the one-dimensional columnar structure, the perovskite can be reused, allowing ammonia to be repeatedly stored and extracted.
An added benefit was that the normally yellow substance turned white following the reaction. Kawamoto claims that “the compound’s ability to change colour when storing ammonia means that color-based ammonia sensors can be developed to determine the amount of ammonia stored.”
There are various applications for the novel storing technology. “In the short term,” says co-author Yoshihiro Ito of RIKEN CEMS, “we hope that this simple and efficient method can be a part of the solution for achieving a decarbonized society through the use of ammonia as a carbon-free hydrogen carrier.”