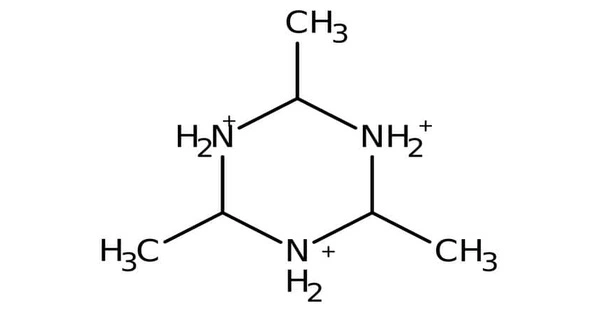
Acetaldehyde ammonia trimer is a chemical compound described by the formula (CH3CHNH)3. The pure material is colorless but samples often appear light yellow or slightly beige due to the degradation by oxidation. It is hygroscopic, and can be found in a trihydrate form.
The acetaldehyde-ammonia trimer is a molecule of interest in organic synthesis, since it can be used as a substrate in many reactions involving acetaldehyde or ammonia. This trimer is well known in the literature but no references are present so far to describe its formation from ammonia sources other than ammonium hydroxide.
Properties
- Chemical formula: C6H15N3
- Molar mass: 129.207 g·mol−1
- Appearance: Colorless crystals
- Melting point: 95 to 97 °C (203 to 207 °F; 368 to 370 K)
- Solubility: polar organic solvents
As implied by its name, it is a trimeric species formed from the reaction of acetaldehyde and ammonia:
3 CH3CHO + 3 NH3 → (CH3CHNH)3 + 3 H2O
Studies using NMR spectroscopy indicate that the three methyl groups are equatorial, thus the molecule has C3v point group symmetry.
The compound is related to hexamethylenetetramine, which is the condensation product of ammonia and formaldehyde.
Applications
Acetaldehyde ammonia trimer is used as scavengers for sulfhydryl compounds in natural gas. It is a reagent used in the synthesis of amines by hydrogenation and as a precursor to the production of ammonia. It can also be used as an organic building block in the synthesis of 1,2-dihydro-1,3-dimethyl-3H-naphth[1.2-e]-m-oxazine by condensing with 2-naphthol.
Safety
Desiccate at 4°C. Store away from air, moisture, and oxidizing agents. Store under dry inert gas. Protect from humidity and water.