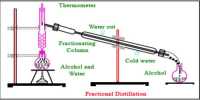
Steam distillation is a special type of distillation (a separation process) for temperature sensitive substance like natural atomic compounds. Many organic compounds tend to decompose at high sustained temperatures. Separation by normal distillation would then not be an option, so water or stem is introduced into the distillation apparatus. By adding water or stem, the boiling points of the compounds are depressed allowing them to distilled are very sensitive to heat, steam distillation can also be combined with vaccum distillation. After distillation the vapours are condensed as usual, usually yielding a two phase system of water and the organic compounds, allowing for decantation.
According to Dalton’s law of partial pressure we see, P = PH2O + Px
PH2O is pressure of liquid vapour and Px is pressure of vapour of volatile component. This is the basic principle of stem distillation.
Description: During steam distillation flow of steam conducts continuously in halt filled flux. It may not necessary is heat the flux perceptibly But distillation flux is heated occasionally so that entered and emitted vapor is equal.
Water is also mixed with organic compound in the collected liquid of the flux. Organic liquid is separated from water by the help of separation funnel and presence of liquid drop is dried by drier materials.
Application:
- Steam distillation is employed in the manufacture of essential oils, for use in perfumes.
- Eucalyptus oil and orange oil are obtained by this method on the industrial scale.
- Steam distillation is also sometimes used to separate intermediate or final products during the synthesis of complex organic compounds.
- Steam distillation is also already used in petroleum refineries and petrochemical plants where it is commonly referred to as steam stripping.