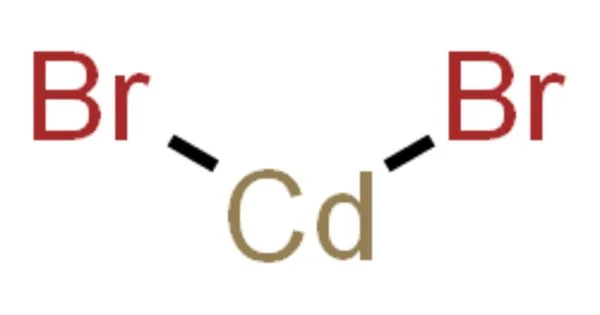
Cadmium bromide is a crystalline powder that ranges in color from white to yellowish. CdBr2 is the formula for an inorganic compound. It is a hygroscopic white solid. It is also available as a mono- and tetrahydrate. It has only a few applications.
Cadmium bromide is used in the engraving, lithography, and photography processes. Cadmium is an element found naturally in the earth’s crust. It is typically found as a mineral in the presence of other elements such as oxygen, chlorine, or sulfur. Cadmium is present in most soils and rocks, including coal and mineral fertilizers. Cadmium is found in cigarette smoke and is used in many products such as batteries, pigments, metal coatings, and plastics. Cadmium enters the environment via mining operations as well as wind and rain. Forest fires and volcanoes also release some cadmium to the air.
Properties
It is white to yellowish powder or flakes; hexagonal crystal system; hygroscopic; density 5.192g/cm3; melts at 568°C; vaporizes at 844°C; soluble in water, alcohol, ether, acetone, and liquid ammonia.
- Chemical formula: CdBr2
- Molar mass: 272.22 g/mol
- Appearance: white solid
- Density: 5.192 g/cm3, solid
- Melting point: 568 °C (1,054 °F; 841 K)
- Boiling point: 844 °C (1,551 °F; 1,117 K)
- Solubility in water: 56.3 g/100 mL (0 °C); 160 g/100 mL (100 °C)
- Solubility: soluble in alcohol, ether, acetone and liquid ammonia.
- Crystal structure: Rhombohedral

Preparation
Cadmium bromide is made by heating cadmium in the presence of bromine vapor. Dry cadmium acetate can be treated with glacial acetic acid and acetyl bromide to produce the compound. It can also be obtained by dissolving cadmium or cadmium oxide in hydrobromic acid and evaporating the solution to dryness in an inert atmosphere under helium.
Uses
This substance is employed in the fields of photography, engraving, and lithography. The other halogen elements combine with cadmium in an ionic reaction similar to bromine.
Health Hazard
Coughing and sneezing are symptoms of lung damage caused by inhalation. Ingestion causes severe toxic symptoms, including kidney and liver damage. Irritation occurs when the product comes into contact with the eyes.