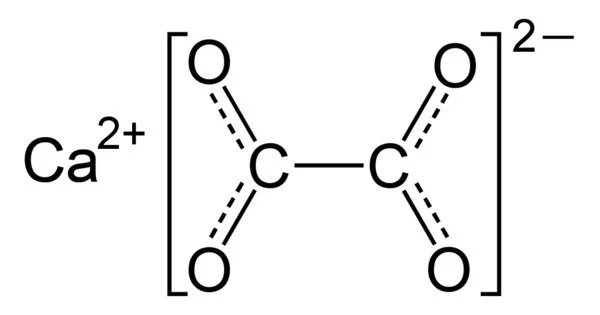
Calcium oxalate, with the chemical formula CaC2O4, is a calcium salt of oxalic acid. It is the calcium salt of oxalic acid, and an excess of it in the urine can result in the formation of oxalate calculi (kidney stones). CaC2O4•nH2O hydrates form, where n ranges from 1 to 3. Colorless or white are the anhydrous and hydrated forms. CaC2O4•H2O occurs naturally as the mineral whewellite, forming envelope-shaped crystals known as raphides in plants.
It is a crystalline salt that is normally deposited in many plant cells and is occasionally excreted in urine or retained in the form of urinary calculi in animals. Some foods contain high levels of calcium oxalates, which can cause sores and numbness when consumed and can even be fatal. Tribes with diets that depend highly on fruits and vegetables high in calcium oxalate, such as in Micronesia, reduce the level of it by boiling and cooking them.
Properties
Calcium oxalate has 128.096 g/mol as its molar mass. It appears colorless or white crystal, whether in anhydrous or hydrated form. The density of monohydrate calcium oxalate is 2.20 g/cm³. Its melting point is 200°C. It means the monohydrate form of calcium oxalate starts decomposing at this temperature. It is highly insoluble in water.
- Chemical formula: CaC2O4
- Molar mass: 128.096 g·mol−1
- Appearance: colorless or white crystals (anhydrous and hydrated forms)
- Density: 2.20 g/cm3, monohydrate
- Melting point: 200 °C (392 °F; 473 K) decomposes (monohydrate)
- Solubility in water: 0.61 mg/100 g H2O (20 °C)
Chemical properties
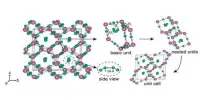
Calcium oxalate is a compound composed of calcium ions and the oxalic acid conjugate base, the oxalate anion. Because of the basicity of the oxalate ion, its aqueous solutions are slightly basic. Because calcium oxalate is less soluble in water, its basicity is weaker than that of sodium oxalate. X-ray crystallography was used to characterize solid calcium oxalate hydrate. It is a polymer of coordination. Planar oxalate anions are linked to calcium and have water ligands.
Occurrence
It has been reported that calcium oxalate accumulates in over 1000 different plant genera. The accumulation of calcium oxalate is linked to the detoxification of calcium (Ca2+) in the plant. When calcium oxalate decomposes, bacteria, fungi, or wildfire oxidize it to produce the soil nutrient calcium carbonate.
Oxalates are found in a wide variety of foods, making it difficult to avoid them. However, the formation of calcium oxalate in the body can result in a variety of complications, such as urinary tract infections, kidney stones, and so on. Certain dietary changes, on the other hand, can help you avoid them.