Explanation characteristics of the photoelectric effect with the help of Einstein Equation
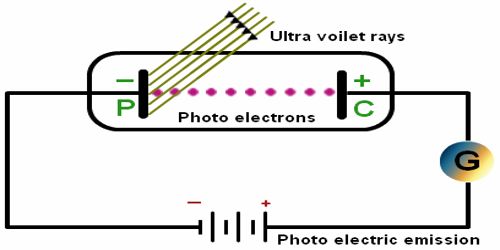
The photoelectric effect refers to the emission, or ejection, of electrons from the surface of, generally, a metal in response to incident light. The photoelectric effect was first observed in 1887 by Heinrich Hertz during experiments with a spark-gap generator (the earliest form of radio receiver).
Einstein’s model is dependable with the surveillance that the electrons were emitted instantaneously when the light was shone on the metal and that the concentration of the light made no dissimilarity to the utmost kinetic energy of the emitted electrons.
Explanations of different characteristics of the photoelectric effect with the help of the Einstein equation (Results obtained by photo-electric action) are given below:
(a) According to this theory, and radiation is the summation of the innumerable photons, the energy of each photon is ‘hv’. So, as the intensity of light increases, a number of photons also increases and hence photoelectric current also increases. But if the frequency of the incident light remains unchanged, then the energy of the photon does not increase, rather the velocity and kinetic energy of the photon remain unchanged. So, quantum theory is in conformity with the experimental result.
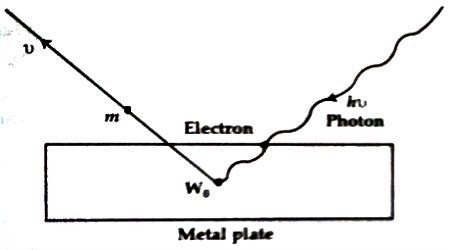
(b) We know, W0 = hv0, is a constant quantity. So from Einstein’s theory, it is seen that the kinetic energy of the electron is directly proportional to the frequency of the incident light.
(c) According to this theory, when a photon collides with an electron the absorbed energy by the electron is not distributed to other electrons. So, in this collision energy is conserved i.e., it is an elastic collision. Again, inelastic collision energy transfer takes place instantaneously. So, an incidence of the rays of light and the emission of electrons occur simultaneously.
(d) As the frequency of light gradually decreases, the velocity of the electron decreases as well and for a minimum frequency u0 the velocity becomes zero. Hence the photoelectron emits. So, for each metal, there exists a minimum frequency which is called threshold frequency. It is denoted by v0. So, quantum theory can explain elaborately the photoelectric effect.
As the frequency increases further than the entrance, the ejected electrons basically shift faster. An augment in the concentration of incoming light that is above the threshold occurrence causes the number of electrons that are ejected to increase, but they do not travel any faster. Einstein proposed that light consisted of quanta, which we call photons. The photoelectric effect is applied in devices called photoelectric cells, which are usually found in everyday items such as a calculator which uses the energy of light to produce electricity.
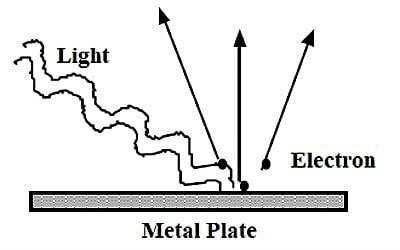
Characteristics of the photoelectric effect –
- For a given metal and frequency of occurrence radiation, the number of photoelectrons turned out per second is straight proportional to the concentration of confrontation radiation.
- The utmost kinetic energy of the emitted photoelectron is autonomous of the concentration of confrontation radiations.
- Light has properties of both a wave and a particle.
- Emission of photoelectrons is instantaneous
- The photoelectric effect is formed by light prominent metal and dislodging electrons from the exterior of the metal.