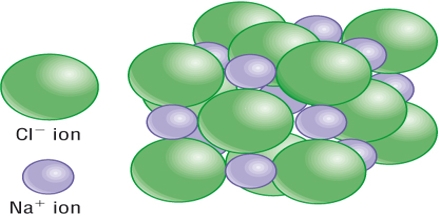
In ionic crystals, the units occupying lattice points are positive and negative ions. Each ion of a given sign is held by coulombic forces of attraction to all ions of opposite sign. The forces are very strong. The ionic crystals have the following characteristics.
- The heats of vaporization of ionic crystals are high.
- The vapour pressure of ionic crystals at ordinary temperature are very low.
- The melting and boiling points of ionic crystals are very high.
- Ionic crystals are hard and brittle.
- Ionic crystals are insulators in the solid state.
- Ionic crystals are soluble in water and also in other polar solvents.
- Ionic solids are good conductors when dissolved in water.