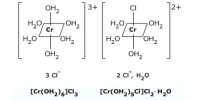
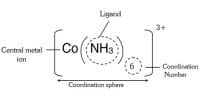
When a ligand is bound to a metal ion through a single donor atom, as with Cl–, H2O or NH3, the ligand is said to be unidentate. Whenever a single coordinating group (or) ligand occupies two (or) more coordination position on the same central metal ions, a complex possessing a closed ring is formed. Such ligands are called polydentate ligands. When a single ligand has two coordinating positions, it is called bidentate ligand and when there are three coordinating positions available, it is called a tridentate ligand and so on.
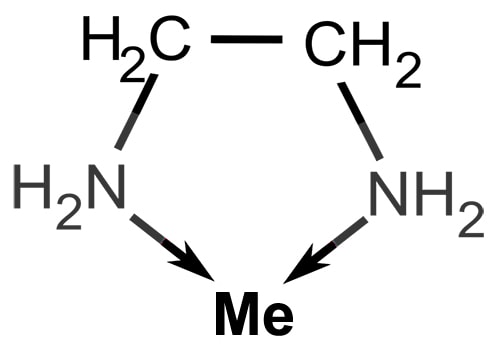
For example, ethylenediamine is a bidentate ligand because it has two amino groups each of which can donate a pair of electrons.
Name of the ligands:
Positive ligands
The positive ligands are named with an ending -ium.
NH2 – NH3+ hydrazinium
This ligand, though positive can bind through the uncharged nitrogen.
Neutral ligands
The neutral ligands are named as such without any special name. But water is written as ‘aqua: Ammonia is written as ammine. Note that two m’s to distinguish from organic amine
CO-Carbonyl, NO-Nitrosyl, NH2 – CH2 – CH2 – NH2-ethylenediamine (en), Pyridine C5H5N.
Negative Ligands
Negative ligands end in suffix ‘O’.
Example
F– -Fluoro, Cl– -Chloro, C2O42- -Oxalato, CN– -Cyano, NO2- -Nitro, Br– -Bromo, SO42--Sulphato, CH3 COO– -acetato CNS– -thiocyanato, NCS– -isothiocyanato, S2O32- -thiosulphato.