Ionisation enthalpy is the energy required to remove a mole of electrons from a mole of free gaseous atoms i.e. the energy required to remove an electron from an atom e.g. Mg (g) 4 Mgt (g) + e-
This is known as the 1st Ionisation Enthalpy. What would affect the ionisation enthalpy? The degree of attraction the outer electrons feel from the nucleus.
First Ionisation Enthalpy
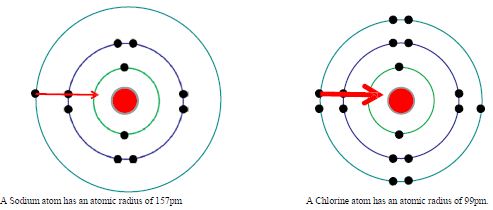
Across a period the ionisation enthalpy increases from left to right. Down a group the first ionisation enthalpy decreases from top to bottom. What affects how much nuclear charge an electron feels? Across a period increase in nuclear charge and decrease of atomic size makes it more difficult to remove an electron. Down a group there is a shielding effect from the extra energy levels, and increased distance from the nucleus makes it easier to remove an electron.
The outermost electron in Sodium is further away from the nucleus than in Chlorine, so is more easily pulled away.
Second Ionisation Enthalpy
The second Ionisation Enthalpy of an element is the energy required to remove the second mole of electrons as for example:
Mg (g) -, Mgt (g) + e-
So the total energy to remove 2 moles of electrons from an atom is the sum of the first and second Ionisation Enthalpies. The second Ionisation energy of an atom is always larger than the first because it involves removing an electron from a species that is already positively charged.