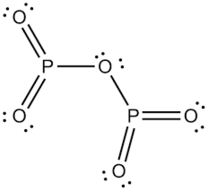
Phosphorus trioxide (P2O3 or P4O6) is obtained by the combustion of phosphorus in a limited supply of air.
4P + 3O2 → 2P2O3
Physical properties
- It is a white waxy substance
- It has a garlic odour.
Chemical properties
- It reacts with cold water, gives phosphorus acid.
P2O3 +3H2O → 2H3PO3
- It reacts with hot water vigorously to form inflammable phosphine.
2P2O3 + 6H2O → PH3 ↑ + 3H3PO4