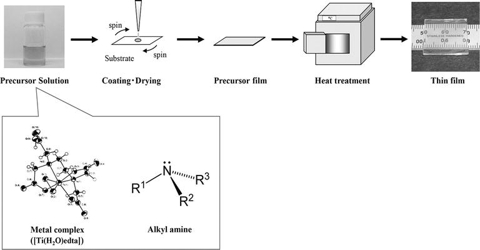
Precursor method: The precursor method is a novel synthesis method for the preparation of solid substances of correct stoichiometry from a single source solid precursor. For example, an oxide MM’2O4 can be prepared if a mixed salt of an acetate containing M and M’ in the ratio 1:2 is formed, which upon heating decomposes to produce the desired compound. Doped semiconductor, nanocrystals encapsulated within oxide-shells, coating metal oxide film on titanium, ultrafine Ni-Zn ferrites etc. have been prepared by the precursor method.
- the precursor is heated to decompose it to the desired product.
- homogeneous products are formed at relatively low temperatures.
- it is not always possible to find a suitable precursor
Precursor method example
Ti(OBu)4 (aq) + 4H2O(l) → Ti(OH)4(s) + 4BuOH(aq)
Excess oxalic acid redissolves the precipitate
Ti(OH)4(s) + (COO)22-(aq) → TiO(COO)2(aq) +2OH–(aq) + H2O(l)
Ba2+(aq) + (COO)22-(aq) + TiO(COO)2(aq) → Ba[TiO((COO)2)2](s)
Decomposition by heating (920 K) gives the desired oxide phase.
Ba[TiO((COO)2)2](s) → BaTiO3(s) + 2CO2(g) + 2CO(g)
Products from precursor methods often contain small particles with a large surface area, which is desired for certain applications.