When the light emitted from a source is made to pass through an absorbing material and then examined with a spectrometer, the obtained spectrum is called absorption spectrum. It is the characteristic of the absorbing substance.
Absorption spectra is also of three types
- continuous absorption spectrum
- line absorption spectrum and
- band absorption spectrum
Continuous absorption spectrum
A pure green glass plate when placed in the path of white light, absorbs everything except green and gives continuous absorption spectrum.
Line absorption spectrum
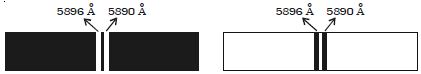
Fig: Emission and absorption spectrum of sodium
When light from the carbon arc is made to pass through sodium vapour and then examined by a spectrometer, a continuous spectrum of carbon arc with two dark lines in the yellow region is obtained as shown in Figure.
Band absorption spectrum
If white light is allowed to pass through iodine vapour or dilute solution of blood or chlorophyll or through certain solutions of organic and inorganic compounds, dark bands on continuous bright background are obtained. The band absorption spectra are used for making dyes.