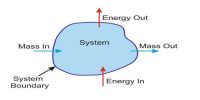
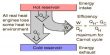
Any heat engine is used to convert heat into mechanical work. An ideal engine free from all in perfections of practical engines was conceived by Sadi Carnot, a French scientist, in 1824. The ideal engine consists of the following essential components:
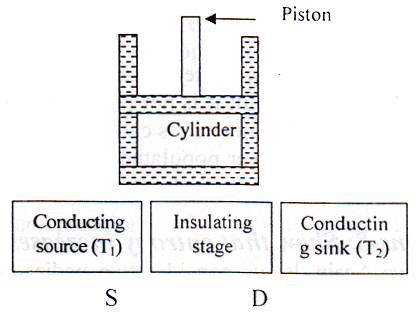
- Cylinder: A perfect gas is used as working substance. The gas is contained in a cylinder with perfectly non-conducting walls, having a frictionless non-conducting piston.
- Source (S): A source or heat reservoir (S) is a thermal system kept at higher temperature T1K of high thermal capacity.
- Sink: The sink (B) is also a thermal system kept at lower temperature T2K, where T1>>T2. It has also very high thermal capacity. It can reject heat.
- Platform: The engine has a platform which is kept on non-conducting cap (D). Since the source and sink are of very high thermal capacity, so due to exchange of heat there will be no change of temperature.
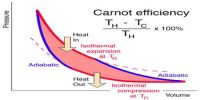
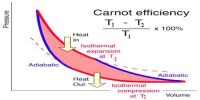
A Carrot’s engine, the working substance under goes two successive thermal expansions and two successive compressions—one is isothermal and other is adiabatic.