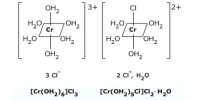
The charge on the complex ion is equal to the sum of the charges on the metal ion and their ligands.
Example
- [Cu(NH3)4]2+ can be written as [Cu2+(NH3)4]2+ since NH3 ligand is neutral.
The sum of the charges on the metal ion and the ligands = +2.
This can be determined as shown below
Charge on the metal ion (Cu2+) = +2
Charge on the ligand (NH3) = 4 × 0 = 0
∴ Net charge on the complex ion = +2 + 0 = +2
- Similarly for [Fe(CN)6]4- (or) [Fe2+ (CN)6]4-
The sum of the charge on the metal ion and the ligand = –4.
Charge on the metal ion (Fe2+) = +2
Charge on the ligand (CN-) = 6 × (-1) = –6
∴ Net charge on the complex = +2 – 6 = –4