Properties of Copper Sulphate (CuSO4.5H2O)
Physical properties
- The anhydrous salt is colourless but the hydrated salt is blue in colour.
- It readily dissolves in water but is insoluble in alcohol.
Chemical Properties
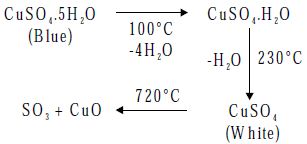
- Action of Heat
On heating CuSO4.5H2O loses its water of crystallization and decomposes at 720°C to give cupric oxide and sulphur trioxide.
- Action of ammonia
Copper sulphate gives deep blue colour with NH4OH forming complex compound.
CuSO4 + 4NH4OH → [Cu (NH3)4]SO4 + 4H2O
- Action of KI
When KI is added to a solution of CuSO4, a white precipitate of cuprous iodide is produced.
CuSO4 + 2KI → Cu I2 + K2SO4 (unstable)
2CuI2 → Cu2I2 + I2 (white ppt)
- Action with KCN
A yellow precipitate of cupric cyanide is first formed with KCN and it decomposes to give cyanogen gas.
CuSO4 + 2KCN → Cu(CN)2 + K2SO4
2Cu(CN)2 → Cu2(CN)2 + (CN)2 (cyanogens)