Diabetes can be exacerbated by the loss or dysfunction of a key type of pancreatic cell known as beta cells. Beta cells are in charge of producing and releasing insulin, a hormone that aids in blood sugar regulation. In people with type 1 diabetes, the immune system mistakenly attacks and destroys beta cells, resulting in severe insulin deficiency.
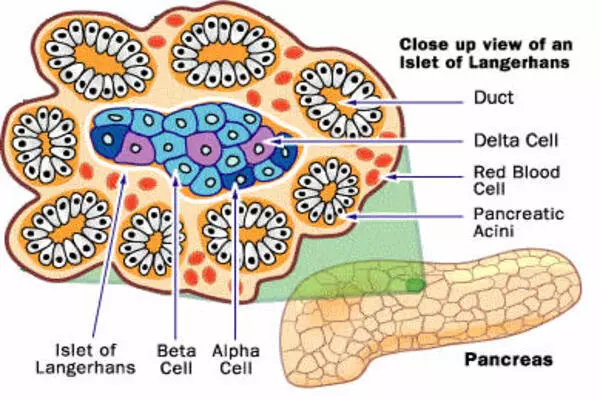
In the pancreas, various types of beta cells produce insulin, which aids in blood sugar regulation. According to Weill Cornell Medicine researchers, losing a particularly productive type of beta cell may contribute to the development of diabetes.
Dr. James Lo, associate professor of medicine at Weill Cornell Medicine, and colleagues measured gene expression in individual beta cells collected from mice in the study, which was published in Nature Cell Biology, to determine how many different types of beta cells exist in the pancreas. The researchers discovered four distinct beta cell types, one of which stood out. Cluster 1 beta cells produced more insulin than other beta cells and appeared to be better at metabolizing sugar. The study also found that the loss of these beta cells may contribute to type 2 diabetes.
“Before this, people thought a beta cell was a beta cell, and they just counted total beta cells,” said Dr. Lo, who is also a member of the Weill Center for Metabolic Health and the Cardiovascular Research Institute at Weill Cornell Medicine and a cardiologist at NewYork-Presbyterian/Weill Cornell Medical Center. “But this study tells us it might be important to subtype the beta cells and that we need study the role of these special cluster 1 beta cells in diabetes.”
Drs. Doron Betel, Jingli Cao, Geoffrey Pitt and Shuibing Chen at Weill Cornell Medicine teamed up with Dr. Lo to carry out the study.
CD63 expression provided us with a way to identify the cells without destroying them and allowed us to study the live cells. They are very high-functioning beta cells. We think they may carry the bulk of the workload of producing insulin, so their loss might have profound impacts.
Drs. Doron Betel
The researchers used a technique known as single-cell transcriptomics to measure all of the genes expressed in individual mouse beta cells before categorizing them into four types. Cluster 1 beta-cells had a distinct gene expression signature, including high expression of genes that aid cellular powerhouses known as mitochondria in breaking down sugar and supplying energy to them so they can secrete more insulin. Furthermore, they were able to distinguish cluster 1 beta-cells from other beta cell types due to the high expression of the CD63 gene, allowing them to use the CD63 protein as a marker for this specific beta cell type.
“CD63 expression provided us with a way to identify the cells without destroying them and allowed us to study the live cells,” he explained.
When the team looked at both human and mouse beta cells, they found that cluster 1 beta cells with high CD63 gene expression produce more insulin in response to sugar than the three other types of beta cells with low CD63 expression.
“They are very high-functioning beta cells,” Dr. Lo said. “We think they may carry the bulk of the workload of producing insulin, so their loss might have profound impacts.”
The number of these insulin-producing powerhouse beta cells decreased in mice fed an obesity-inducing, high-fat diet and in mice with type 2 diabetes. “Because the number of cluster 1/high CD63 cells decreased, you may have less insulin production, which may play a significant role in the development of diabetes,” he explained.
Transplanting beta cells with high CD63 production into type 2 diabetes mice restored normal blood sugar levels. When the transplanted cells were removed, the high blood sugar levels returned. Transplanting low CD63 production beta cells into mice did not return blood sugar levels to normal. Instead, the transplanted low CD63 beta cells appeared dysfunctional.
According to Dr. Lo, the discovery could have significant implications for the use of beta cell transplants to treat diabetes. It may be preferable, for example, to transplant only high CD63-beta cells. He also suggested that fewer of these highly productive cells could be transplanted. Dr. Lo’s team also discovered that people with type 2 diabetes had lower levels of high CD63 beta cells than those who did not have diabetes.
Dr. Lo and his colleagues want to know what happens to the high CD63-producing beta cells in diabetic mice and how to keep them from disappearing. “If we can figure out how to keep them around longer, surviving and functional, that could lead to better ways to treat or prevent type 2 diabetes,” he said.
They also want to look into how current diabetes treatments affect all types of beta cells. GLP-1 agonists, which help increase insulin release in diabetics, interact with both high and low CD63-producing beta cells.
“Our findings also suggest that GLP-1 agonists could be used to improve the function of low CD63-producing beta cells,” Dr. Lo said.