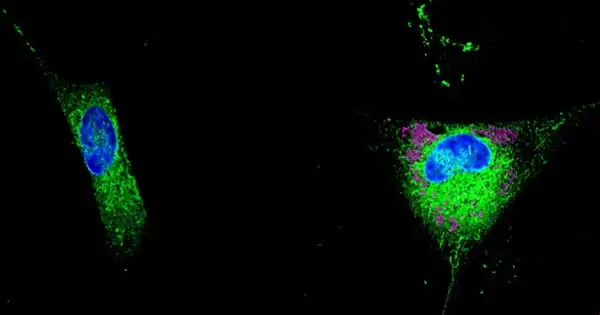
Tens of billions of neurons—specialized cells that process and send information via electrical and chemical signals—are found in a healthy human brain. They communicate between different areas of the brain as well as between the brain and the muscles and organs of the body. Alzheimer’s disease affects this connection between neurons, resulting in functional loss and cell death.
Neurons in the brain cohabit with and rely on a variety of different cell types in order to operate effectively. Astrocytes, which get their name from their star-like form, feed and detoxify neurons with the help of a multipurpose protein called APOE. APOE4, one of three versions of this protein, greatly raises the risk of developing Alzheimer’s disease, but the mechanisms involved remain unknown.
A team of researchers from the University of Geneva (UNIGE), the European Molecular Biology Laboratory (EMBL), the University of Zurich, and the pharmaceutical company AbbVie uncovered a plausible mechanism: rather than stopping to function, APOE4 is becoming more efficient. It produces the accumulation of potentially hazardous lipids that are damaging to neurons by stimulating astrocytic lipid release, and hence may contribute to the development of Alzheimer’s disease. These findings, which were published in the journal Cell Reports, give fresh light on the neurodegenerative mechanisms behind a disease that affects almost 50 million people globally.
We discovered that cellular aging redirects APOE from its primary job of transporting lipids to neurons and recovering lipid waste from them to the release of triglycerides, which are specific lipid species that can be detrimental if not eliminated.
Karina Lindner
Astrocytes, which are abundant in the brain, play an important protective role. These cells release apolipoprotein E (or APOE), a tiny protein that creates particles containing fats and vitamins to feed the neurons. It also detoxifies the neurons by removing “lipid waste” that could be detrimental if not removed. Because neurons are unable to clear this waste on their own, APOE steps in to collect it and transport it to the astrocytes, where it is destroyed.
The APOE gene has three common variants in humans: APOE2, which is found in 8% of the population, APOE3, which is the most prevalent, and APOE4, which is found in nearly 15% of people and raises the chance of getting Alzheimer’s disease by a factor of 10. “The reasons why APOE4 increases the risk of Alzheimer’s disease so significantly are not well understood,” says Anne-Claude Gavin, a professor in the Department of Cell Physiology and Metabolism at the UNIGE Faculty of Medicine and holder of a Louis-Jeantet Foundation Chair, who led this research with Viktor Lakics, a Research fellow and Biology Area Leader in Neuroscience discovery at AbbVie.
What are the mechanisms underlying APOE4 dysfunction? Above all, might they be used as a foundation for prevention or therapy? Anne-Claude Gavin and her colleagues collaborated with scientists from the European Molecular Biology Laboratory (EMBL), the University of Zurich, and AbbVie to find answers to these issues.
A protein that is too effective
Working to answer these issues, the researchers discovered new chemical pathways that explain how APOE interacts to astrocyte membranes to identify and extract the lipids it requires. In vitro investigations using human cell lines with different APOE variants revealed that APOE is particularly efficient at transferring potentially hazardous lipids generated in neurons.
“And, to our great surprise, the APOE4 variant proved to be even more efficient than the other forms,” says Katharina Beckenbauer, a former postdoctoral researcher in Anne-Claude Gavin’s lab, senior scientist at AbbVie, and one of the study’s first authors. “So, according to popular belief, the problem is not that APOE4 ceases to function, but rather the inverse. And the machine malfunctions.”
A hijacked function
As astrocytes age, their efficiency decreases and they begin to collect lipids rather than eliminate them. “We modelled this process experimentally and saw the chemicals released by the astrocytes,” Karina Lindner, a PhD student in Anne-Claude Gavin’s laboratory and one of the study’s original authors, explains. “We discovered that cellular aging redirects APOE from its primary job of transporting lipids to neurons and recovering lipid waste from them to the release of triglycerides, which are specific lipid species that can be detrimental if not eliminated.”
This problem is aggravated by APOE4: it promotes triglyceride secretion, resulting in their excessive buildup. This hazardous accumulation of potentially dangerous lipids could very well be a key contributor to neuronal death, a characteristic of Alzheimer’s disease. “APOE4 would thus have the power to accelerate the degenerative process in the disease through the mechanism we have uncovered.”
To better understand the nuances of APOE’s function, particularly the E4 version, researchers at UNIGE want to know how the release of these potentially damaging lipids is regulated and whether this secretion can be identified in persons with Alzheimer’s disease.