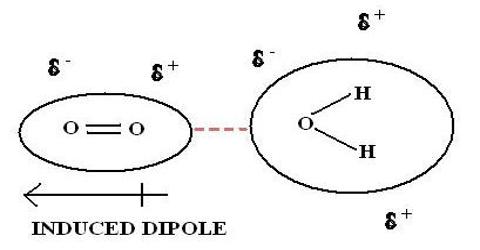
Dipole-induced dipole interactions:
Dipole-induced dipole interaction involves the attraction between temporally induced dipoles in non-polar molecules. This polarization can be induced either
(a) by a polar molecule or
(b) by the repulsion of the negatively charged electron clouds in a non-polar molecule.
An example of the former is chlorine dissolving in water.
[Permanent dipole) H – 0 – H – – – – Cl – Cl [Induced dipole]
This is an example of interaction between the permanent dipole of water molecule and an induced dipole on chlorine molecule. The dipole in non-polar chlorine molecule is induced by the electric field offered by the permanent dipole of water molecule. This permanent dipole -induced dipole interaction is referred to as induction (or polarization) interaction and is to be distinguished from London dispersion interaction.