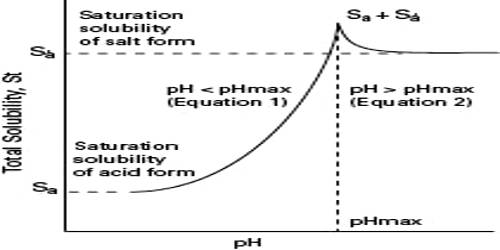
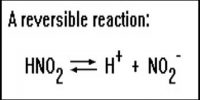Effect of pH on Solubility
When one or more of the ions in a solubility equilibrium is derived from a weak acid or a weak base, the solubility of the compound is profoundly affected by pH. For example, let us consider the case of Mg(OH)2. The solubility equilibrium for this hydroxide is
Mg(OH)2 + aq ↔ Mg2+ (aq) + 2OH– (aq)
The value of Ksp for Mg(OH)2 is 1.8 x 10-11. Suppose that solid Mg(OH)2 is placed in a buffer solution of pH = 9. The solution has a pOH of 5. i.e., in the solution [OH–] = 1 x 10-5. Inserting this value of [OH–] in the expression of the solubility product-
Ksp = [Mg2+] [OH–]2 = 1.8 x 10-11
= [Mg2+] [1.8 x 10-11] = 1.8 x 10-11
= [Mg2+] = 1.8 x 10-1 = 0.18 mol L-1.
Thus Mg(OH)2 is quite soluble in slightly alkaline solution. If the solution was made more acidic the [OH–] will decrease and the solubility will increase because the [Mg2+] has to increase in order to maintain equilibrium.
On the contrary, an addition of H3O+ to a saturated solution at a compound with an anion of the strong acid will have no effect on the equilibrium position. As an example, let us consider the following equilibrium:
AgCl(s) ↔ Ag+ (aq) + Cl– (aq)
Here, Cl– ion, a conjugate base of a strong acid, HCl, can co-exist in solution with high H3O+. The Cl– ion does not leave the solution, so equilibrium position is not affected.