Water electrolysis is the use of electricity to split water into oxygen (O2) and hydrogen (H2) gas. It is a chemical process that splits water molecules (H2O) into their constituent elements, hydrogen (H2) and oxygen (O2). This occurs in an electrolysis cell, which typically consists of two electrodes immersed in a water-based electrolyte solution.
The hydrogen gas produced in this manner can be used as hydrogen fuel, but it must be kept separate from oxygen because the mixture would be extremely explosive. Because the hydrogen/oxygen flame can reach around 2,800°C, hydrogen can be pressurised separately and stored in convenient ‘tanks’ or ‘gas bottles’ for use in oxyhydrogen welding and other applications.
Water electrolysis requires a minimum potential difference of 1.23 volts, though external heat is also required at that voltage. Typically, 1.5 volts are needed. Because hydrogen can be produced more cheaply from fossil fuels, electrolysis is rarely used in industrial applications.
Here’s how the electrolysis of water works:
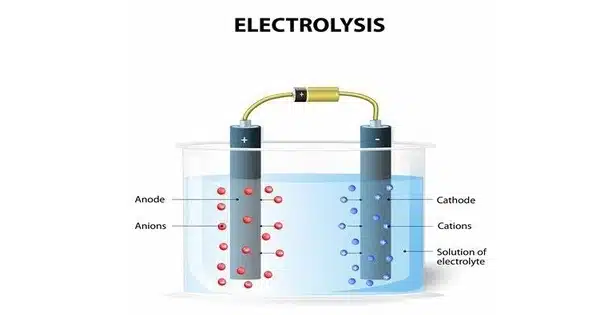
(a) Setup: Two electrodes made of conductive materials, such as platinum or graphite, are immersed in a container of water to form an electrolysis cell. An external power source, such as a battery or a power supply, is connected to the electrodes.
(b) Electrolyte: A small amount of sulfuric acid (H2SO4) or potassium hydroxide (KOH) is sometimes added to the water as an electrolyte. The presence of an electrolyte increases the conductivity of the water and increases the efficiency of the process.
(c) Electrolysis: When an electric current is passed through the water between the two electrodes, several reactions occur at the electrodes:
- At the cathode (negative electrode), water molecules near the electrode gain electrons and are reduced, forming hydrogen gas (H2): 2H2O + 2e⁻ → H2(g) + 2OH⁻
- At the anode (positive electrode), water molecules near the electrode lose electrons and are oxidized, forming oxygen gas (O2): 2H2O → O2(g) + 4H⁺ + 4e⁻
(d) Collection of Gases: The hydrogen gas and oxygen gas produced during the electrolysis are collected separately. They can be collected over water to prevent contamination by air.
(e) Measurement and Purity: The collected gases can be measured, and their purity can be assessed through various methods, such as the flame test for hydrogen (it burns with a “pop” sound) and the ability of oxygen to support combustion.
The electrolysis of water has several practical applications:
- Hydrogen Production: It is a common method for producing hydrogen gas, which is used in various industries and as a clean energy source in fuel cells.
- Oxygen Production: Oxygen gas produced during this process can be used for various purposes, including medical applications, welding, and as a feedstock in chemical processes.
- Energy Storage: Electrolysis can be used to store excess electricity generated from renewable sources (e.g., wind or solar) by converting it into hydrogen, which can be later used to generate electricity or as a fuel.
Overall, the electrolysis of water is a fundamental process with significant implications in various fields, including energy production, industry, and environmental management.