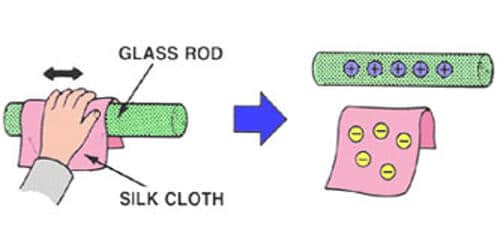
In normal conditions the number of protons and electrons in an atom is equal. But every atom has got an affinity for getting excess electrons. This affinity for an excess electron is different in different substances. That is why when two bodies are brought in contact with each other, the body which has a greater affinity for electron collect electrons from other bodies and gets charged negatively this happens when a glass rod is rubbed with silk. The frictional charging process results in a transfer of electrons between the two objects that are rubbed together. Having an excess of electrons, the rubber balloon is charged negatively. Similarly, the shortage of electrons on the animal fur leaves it with a positive charge.
Example – When a glass rod is rubbed with a silk cloth some electrons from the glass attach themselves to the silk. Consequently, the glass becomes positively charged and the silk negatively charged. Likewise when ebonite is rubbed with fur electrons are transferred from fur to ebonite, thus making the ebonite negative and the fur positive.
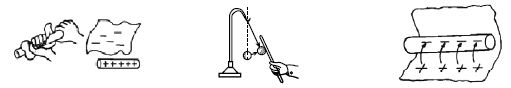
[Fig: 1] [Fig: 2] [Fig: 3]
[Fig: 1] When a glass rod is rubbed with a silk cloth some electrons from the glass attach themselves to the silk. Silk has more electron affinity than glass and as such these two are rubbed together, the electron of glass goes to silk. As a result, the silk gets charged negatively and the glass rod becomes positively charged. This is why the glass rod attracts pith ball [Fig; 2].
Again when a rod of ebonite or polythene is rubbed with flannel, polythene rod gets charged negatively and the flannel becomes positively charged. Consequently, the glass becomes positively charged and the silk negatively charged. Likewise when ebonite is rubbed with fur electrons are transferred from fur to ebonite, thus making the ebonite negative and the fur positive. Because polythene has more affinity for electrons than that of flannel and so when they are rubbed together free electrons of flannel moves to ebonite or polythene and gets charged negatively [Fig: 3].
Why this happens, and why the electrons go from glass to silk and from fur to ebonite and not in the reverse direction are not at present understood. Under normal conditions, the ‘intrinsic’ electric field of an insulator is compensated for by ions absorbed from the air. The friction of one body against another mixes their surface layers so that the compensation is disturbed, i.e. the bodies become electrified.