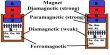
Paramagnetic substances are those in which each atom or molecule has a net non-zero magnetic moment of its own.
- Susceptibility has a low positive value. (For example: Xm for aluminium is +0.00002).
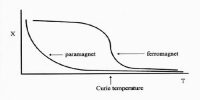
- Susceptibiltity is inversely proportional to absolute temperature (i.e) X α 1/T. As the temperature increases susceptibility decreases.
- The relative permeability is greater than one.
- When placed in a non uniform magnetic field, they have a tendency to move from weaker part to the stronger part of the field. They get magnetised in the direction of the field as shown in Figure.
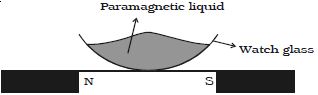
- When suspended freely in a uniform magnetic field, they set themselves parallel to the direction of magnetic field (Figure).

Examples: Al, Pt, Cr, O2, Mn, CuSO4 etc.