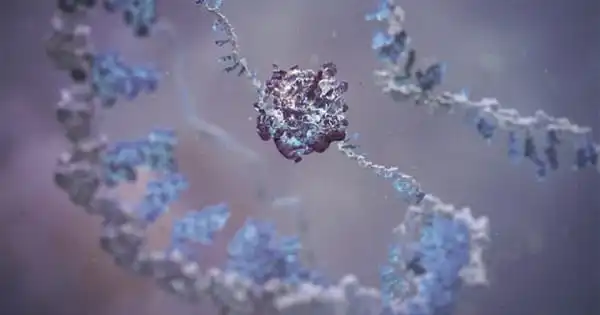
Foam cells are a type of macrophage that resides in fatty deposits on blood artery walls, where they consume low-density lipoproteins and become lipid-laden, giving them a foamy look. These cells emit a variety of chemicals that contribute to plaque formation, and their demise causes inflammation, contributing to cardiovascular disease.
A heart attack or stroke might catch someone off guard who thought they were safe. Now, UConn Health-led research has discovered a novel model that could improve how we monitor cardiac disease. The study was published in Circulation on January 18th.
Heart attacks and strokes are the leading causes of death in the United States, with cholesterol plaques obstructing blood vessels accounting for 70% of all cases. Despite the prevalence of cardiovascular illness, our best efforts to forecast who is at risk of catastrophic events such as a stroke or heart attack remain inadequate. The current risk models take into account a person’s body mass, waist circumference, and the percentage of different fats in their blood. However, it is difficult to use that model to correctly forecast if someone will have a heart attack in the next five years, for example. Many scholars believe that other variables must be at play.
We started looking at macrophages, which are cells that devour lipids adhering to the walls of blood vessels. They appear foamy because the fat droplets are confined within the cell and appear as a clump of bubbles.
Beiyan Zhou
“We started looking at macrophages, which are cells that devour lipids adhering to the walls of blood vessels,” says Beiyan Zhou, an immunologist at UConn Health School of Medicine. Macrophages consume fatty deposits on artery walls. They grow frothy after digesting the fat. “They appear foamy because the fat droplets are confined within the cell and appear as a clump of bubbles,” Zhou explains.
Cardiologists have a low opinion of foamy macrophages. They are frequently observed in plaques along irritated blood vessel walls. Foamy plaque is the most dangerous type of plaque, and it is linked to advanced cardiovascular disease.
However, foamy macrophages may be more neutral and intriguing than they are currently given credit for. Zhou and colleagues from the MultiEthnic Study of Artherosclerosis (MESA) examined the foamy cells in greater detail to see how their lipid consumption connected to heart attacks, arthritis, and even irritable bowel syndrome.

The researchers discovered that foamy macrophages are primarily a cleanup crew that eats fat spread in the wrong areas. Cardiovascular disease worsens if macrophages are prevented from clearing up. However, lipid-eating macrophages function differently in patients with diabetes, autoimmune illness, or who use particular immunotherapies. They continue to consume lipids, but they also get inflammatory, which can aggravate plaques in the arteries and possibly cause ruptures, which result in bits of plaque traveling down the bloodstream until they catch somewhere else, potentially producing a clog.
“Foaming is not always harmful; for the first time, we can discriminate between good and bad foaming. It is the excessive frothing that can lead to a heart attack” Zhou explains.
MESA is a long-term study that Zhou and her colleagues are conducting in collaboration with UConn Health cardiologists Annabelle Rodriguez-Oquendo and Patrick Murphy, as well as immunologist Anthony Vella. They’re looking for the crucial switch that causes foamy cells to become unhealthy and cause disease. MESA provided the team with access to a big dataset of persons who developed heart disease or did not acquire heart disease over the previous 20 years. The database tracks monocytes, which are progenitors of macrophages, as well as the genetic origins of individuals.
MESA data enabled the authors to design a 30 gene model from a pool of poor foaming genes identified by AtheroSpectrum, a tool developed by Zhou’s team. When combined with clinical data on blood pressure, current medications, and diabetes status, the model provided a remarkably accurate score of a person’s risks of having a stroke or heart attack in the following 5 years. Their score outperformed cardiologists’ existing best practices in predicting adverse outcomes.
Profiling the 30 genes expressed by patients’ monocytes is not an easy test to order right now for a cardiologist. But Zhou and her colleagues argue that it should be. Zhou claims that “understanding pathogenic foaming has provided fresh methodologies” for developing future risk assessments for persons with heart disease. “This could potentially provide a gene pool for medication creation precisely targeting harmful foaming in macrophages,” she says.