Fuel cells become a great alternative for hydrogen vehicles and longer-lasting
For truly sustainable mobility, hydrogen is a fuel that cannot be ignored. Fuel cells are gaining in importance as an alternative to battery-operated electromobility in heavy traffic, especially since hydrogen is a CO2-neutral energy carrier if it is obtained from renewable sources. Most manufacturers are experimenting with the technology and some have started producing small numbers of vehicles, but we’re still years from a mass roll-out. For efficient operation, fuel cells need an electrocatalyst that improves the electrochemical reaction in which electricity is generated. The platinum-cobalt nanoparticle catalysts used as standard today have good catalytic properties and require only as little as necessary rare and expensive platinum. A common method of hydrogen production involves separating it from natural gas, but work is underway to obtain hydrogen from biomass, a process that would significantly cut the life-cycle emissions from hydrogen to around 60g/km CO2.
The skeptics’ first argument against hydrogen vehicles is that they’re less efficient than EVs are. Because hydrogen doesn’t occur naturally, it has to be extracted, then compressed in fuel tanks. It then has to mix with oxygen in a fuel cell stack to create electricity to power the car’s motors. In order for the catalyst to be used in the fuel cell, it must have a surface with very small platinum-cobalt particles in the nanometer range, which is applied to a conductive carbon carrier material. Since the small particles and also the carbon in the fuel cell are exposed to corrosion, the cell loses efficiency and stability over time. The key to encouraging hydrogen vehicles is making them part of a wider ‘hydrogen economy’ – building refueling stations for hydrogen cars alone would be inefficient.
An international team led by Professor Matthias Arenz from the Department of Chemistry and Biochemistry (DCB) at the University of Bern has now succeeded in using a special process to produce an electrocatalyst without a carbon carrier, which, unlike existing catalysts, consists of a thin metal network and is, therefore, more durable. “The catalyst we have developed achieves high performance and promises stable fuel cell operation even at higher temperatures and high current density,” says Matthias Arenz. The results have been published in Nature Materials. The study is an international collaboration between the DCB and, among others, the University of Copenhagen and the Leibniz Institute for Plasma Science and Technology, which also used the Swiss Light Source (SLS) infrastructure at the Paul Scherrer Institute.
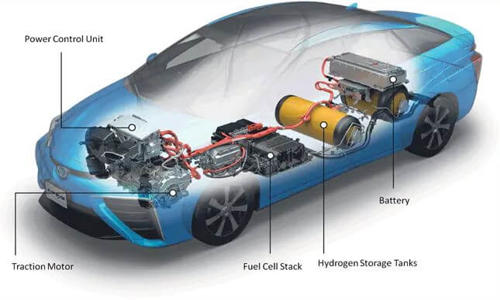
Fig: a hydrogen vehicle
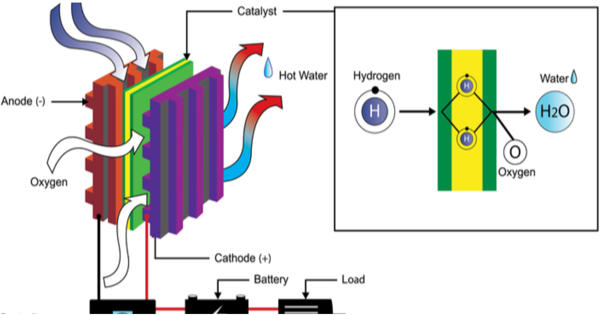
The fuel cell – direct power generation without combustion
In a hydrogen fuel cell, hydrogen atoms are split to generate electrical power directly from them. For this purpose, hydrogen is fed to an electrode, where it is split into positively charged protons and negatively charged electrons. The electrons flow off via the electrode and generate an electric current outside the cell, which drives a vehicle engine, for example. The protons pass through a membrane that is only permeable to protons and react on the other side on a second electrode coated with a catalyst (here from a platinum-cobalt alloy network) with oxygen from the air, thus producing water vapor. This is discharged via the “exhaust.”
The important role of the electrocatalyst
Hydrogen (H2) is an alternative fuel that can be produced from diverse domestic resources. For the fuel cell to produce electricity, both electrodes must be coated with a catalyst. Without a catalyst, the chemical reactions would proceed very slowly. This applies in particular to the second electrode, the oxygen electrode. However, the platinum-cobalt nanoparticles of the catalyst can “melt together” during operation in a vehicle. This reduces the surface of the catalyst and therefore the efficiency of the cell. In addition, the carbon normally used to fix the catalyst can corrode when used in road traffic. This affects the service life of the fuel cell and consequently the vehicle. “Our motivation was, therefore, to produce an electrocatalyst without a carbon carrier that is nevertheless powerful,” explains Matthias Arenz. Previous, similar catalysts without a carrier material always only had a reduced surface area. Since the size of the surface area is crucial for the catalyst’s activity and hence its performance, these were less suitable for industrial use. The goal for vehicle users is to have clean mobility whilst still arriving quickly and comfortably at their destinations. Hydrogen mobility delivers precisely this: effectively, it is electric mobility with the refueling convenience of an ICE.