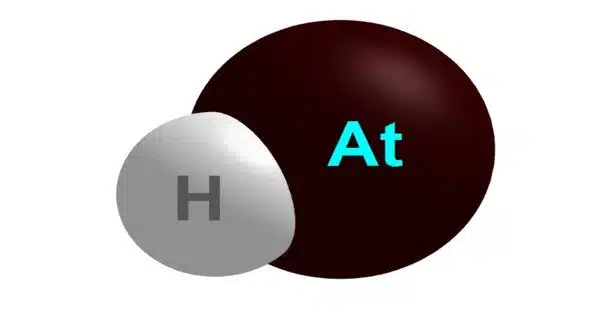
The chemical compound HAt, also known as astatine hydride, astatane, astatidohydrogen, or hydroastatic acid, is composed of an astatine atom covalently bonded to a hydrogen atom. As a result, it is a hydrogen halide.
This chemical compound can dissolve in water to form hydroastatic acid, which has properties similar to the other five binary acids and is the most powerful of them all. However, its use is restricted due to its easy decomposition into elemental hydrogen and astatine, as well as the short half-lives of the various astatine isotopes.
Properties
- Chemical formula: HAt
- Molar mass: 211 g/mol
- Boiling point: −3 °C (27 °F; 270 K) estimated
- Solubility in water: Soluble
- Conjugate acid: Astatonium
- Conjugate base: Astatide
The chemical properties and behavior of hydrogen astatide are similar to other hydrogen halides like hydrogen fluoride (HF), hydrogen chloride (HCl), hydrogen bromide (HBr), and hydrogen iodide (HI). It is a colorless gas at room temperature, but its exact physical properties are not well-studied due to its rarity and instability.
Preparation
Hydrogen astatide can be produced by reacting astatine with hydrocarbons (such as ethane):
C2H6 + At2 → C2H5At + Hat
It’s a binary compound made up of hydrogen (H) and astatine (At), the rarest naturally occurring element on the planet. Because astatine is a highly radioactive element, hydrogen astatide is a highly unstable and reactive compound.
Hydrogen astatide is not found in large quantities in nature due to its high reactivity and radioactivity. It is mostly created in laboratories for research purposes or in trace amounts by the decay of other radioactive isotopes. The compound is a strong acid, and it is regarded as one of the most potent acids known.
Application
Due to its highly reactive and radioactive nature, hydrogen astatide is primarily used in scientific research and not in practical applications. It’s been used in studies on astatine chemistry, radioisotope labeling, and radiopharmaceutical development. However, its scarcity and the difficulties associated with handling astatine have limited its widespread use.