Hydrogen ion concentration- The pH Scale
An accurate knowledge of the hydrogen ion concentration and its control is of utmost importance in many chemicals, analytical, industrial, and biological processes. The hydrogen ion concentration generally found in many chemical and biological systems are very small, and often in the range of 10-2 to 10-12 mol L-1. It is rather difficult to always deal with such small numbers. In order to avoid this inconvenience Soren Peter Lauritz Sorensen (1909), a Danish biochemist proposed a more practical measure of the concentration of hydrogen ion in solutions. He introduced the term pH and defined it as “pH of a solution is the negative logarithm (to the base 10) of the hydrogen ion (strictly speaking H3O+) concentration in mol L-1)”.
pH = – log10 [H3O+] = log 1[H+] … … … (1)
For example the pH of a Solution having a hydrogen ion concentration of 10-3 mol L-1 is-
pH = – log10 (10-3) = 3
[Note: With the introduction of the concept of activity the correct definition is
pH = – log aH+
where aH+, is the activity of hydrogen ion in solution and this should be used inaccurate work. For ordinary purposes the definition of Sorensen is adequate and in all descriptions, concentration will be used in defining pH.]
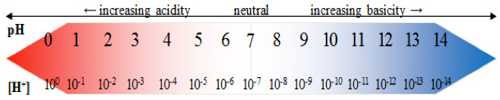
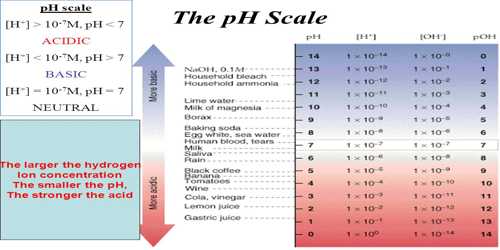
The pH is a dimensionless quantity and its values indicate acidity, basicity and neutral nature of any medium. At 298 K the pH scale ranges from 0 – 14.
- Acidic solution [H+] > 1.0 x 10-7 mol L-1 pH < 7.00
- Basic solution [H+] < 1.0 x 10-7 mol L-1 pH > 7.00
- solution [H+] < 1.0 x 10-7 mol L-1 pH > 7.00
The pH values of some common fluids are given in Table.
Table: pH of some common fluids at 298 K
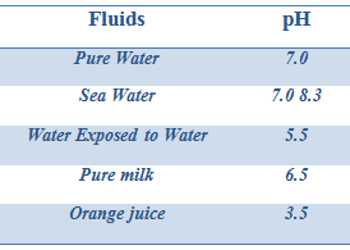
It can be seen from Table, the pH of body fluids varies greatly depending on the locations and metabolic functions that a particular body – part performs. The low pH (high acidity) in the stomach helps in digestion enhances the activity of certain digestive enzymes and transport of H+ ions across the cell membrane. The high pH (basicity) is required for active transport of oxygen.