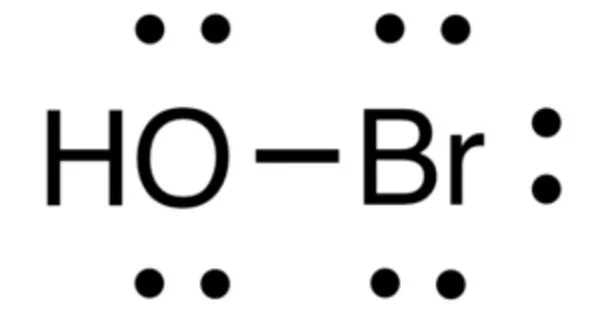
Hypobromous acid is a weak, unstable acid with the chemical formula HOBr. It is a weak and unstable acid with similar chemical and physical properties to hypohalites. Hypohalites are oxyanion with halogen in oxidation state +1. It is primarily produced and handled in an aqueous solution. It is produced both biologically and commercially as a disinfectant. Hypobromite salts are seldom isolated as solids.
Because hypobromous acid has the ability to kill pathogens and bacteria, it is used as a disinfectant, oxidizer, bleach, and deodorant. Because of its ability to kill and complete the cells of many microorganisms, HOBr is effective as a bleach, oxidant, deodorant, and disinfectant. Furthermore, the chemical is produced by warm-blooded vertebrate organisms, particularly eosinophils.
Properties
Hypobromous acid is a chemical compound with a molecular weight of 96.911 g/mol. Furthermore, the density of hypobromous acid (HBrO), a chemical substance, is 2.470 g/cm. It has a pKa of 8.65, which indicates how acidic it is. Its boiling point is between 20 to 25° Celsius.
- Chemical formula: HOBr
- Molar mass: 96.911
- Density: 2.470 g/cm3
- Boiling point: 20–25 °C (68–77 °F; 293–298 K)
- Acidity (pKa): 8.65
- Conjugate base: Hypobromite
Synthesis and properties
Addition of bromine to water gives hypobromous acid and hydrobromic acid (HBr) via a disproportionation reaction.
Br2 + H2O ↔ HOBr + HBr
In nature, hydrobromous acid is produced by bromoperoxidases, which are enzymes that catalyze the oxidation of bromide with hydrogen peroxide:
Br– + H2O2 ↔ HOBr + OH–
Hypobromous acid has a pKa of 8.65 and is therefore only partially dissociated in water at pH 7. Like the acid, hypobromite salts are unstable and undergo a slow disproportionation reaction to yield the respective bromate and bromide salts.
3 BrO–(aq) → 2 Br–(aq) + BrO–3(aq)
Its chemical and physical properties are similar to those of other hypohalites.
Uses
Due to its ability to kill the cells of many pathogens, HOBr is used as a bleach, oxidizer, deodorant, and disinfectant. The compound is produced by warm-blooded vertebrate organisms, particularly eosinophils, via the action of eosinophil peroxidase, an enzyme that preferentially uses bromide.
Bromide is also used as a germicidal agent in hot tubs and spas, using the action of an oxidizing agent to generate hypobromite in the same way that peroxidase in eosinophils does. It works best when combined with its congener, hypochlorous acid.