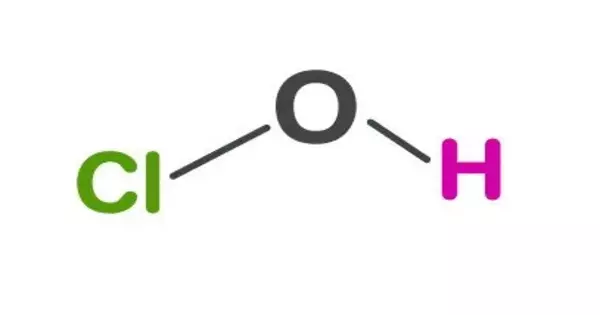
A hypohalous acid is an oxyacid consisting of a hydroxyl group single-bonded to any halogen. Examples include hypofluorous acid, hypochlorous acid, hypobromous acid, and hypoiodous acid. The conjugate base is a hypohalite. They can be formed by reacting the corresponding diatomic halogen molecule (F2, Cl2, Br2, I2) with water in the reaction:
X2 + H2O ⇌ HXO + HX
This also results in the corresponding hydrogen halide, which is also acidic.
Hypohalous acids are powerful oxidizing agents and are known for their antimicrobial properties. Hypochlorous acid, in particular, plays a crucial role in the human immune system as it is produced by specialized cells to kill invading microorganisms. It is effective against bacteria, viruses, and fungi.
Properties
- Chemical Structure: These acids have the general chemical formula HXO, where X represents a halogen atom (chlorine, bromine, or iodine). The halogen atom is directly bonded to an oxygen atom, which is further bonded to a hydrogen atom.
- Acidic Nature: These acids are weak acids. They can dissociate in water to release hydrogen ions (H+) and the corresponding hypohalite ions (XO-). The degree of dissociation varies depending on the specific acid and its concentration.
- Oxidizing Properties: These acids are powerful oxidizing agents. They readily accept electrons from other substances, leading to oxidation reactions. This property makes them effective disinfectants and bleaching agents.
- Disproportionation: These acids undergo spontaneous disproportionation reactions in water, where they simultaneously act as both oxidizing and reducing agents. For example, hypochlorous acid can disproportionize to form chloride ions (Cl-) and chlorate ions (ClO3-).
- Antimicrobial Activity: Hypohalous acids, especially hypochlorous acid, exhibit strong antimicrobial properties. They can kill a wide range of microorganisms, including bacteria, viruses, fungi, and protozoa. Hypochlorous acid is produced by the human immune system to combat infections.
Stability
Hypohalous acids tend to be unstable. Only hypofluorous acid has been isolated as a solid, and even it is explosive at room temperature. Hypochlorous acid cannot be prepared in anhydrous form. Hypobromous acid, hypoiodous acid, and their conjugate bases (hypobromite and hypoiodite) are also unstable, undergoing disproportionation reactions like
3 BrO−(aq) → 2 Br−(aq) + BrO−3(aq)
and
3 HIO → 2 HI + HIO3
that result in the corresponding hydrogen halides/halide ions and halic acids/halates.
Uses
Hypochlorous acid and hypobromous acid are each dissolved in water in order to sanitize it, hypochlorous acid in swimming pools and hypobromous acid in hot tubs and spas. These can also be used as disinfectants and sanitizers due to their antimicrobial properties. They are commonly employed in water treatment, swimming pools, and medical settings for their ability to kill pathogens.