Non-alcoholic fatty liver disease (NAFLD) is characterized by fat accumulation in the liver that is not caused by alcohol consumption. NAFLD can progress to a more severe form known as non-alcoholic steatohepatitis (NASH), which is associated with liver inflammation and can lead to fibrosis, cirrhosis, and liver cancer in some cases.
Researchers discovered novel candidate drug targets for non-alcoholic fatty liver disease (NAFLD) using cutting-edge technologies such as single-nuclear sequencing of mice and human liver tissue and advanced 3D glass imaging of mice to characterize key scar-producing liver cells. The study was led by researchers at Mount Sinai’s Icahn School of Medicine.
Using these novel techniques, the researchers discovered a network of cell-to-cell communication that drives scarring as liver disease progresses. The findings, which were published online on Science Translational Medicine, have the potential to lead to new treatments.
NAFLD is a worldwide threat characterized by fat in the liver and is frequently associated with type 2 diabetes, hypertension, and elevated blood lipids. In the United States, it is estimated that 30 to 40% of adults are affected, with approximately 20% of these patients having a more advanced stage known as non-alcoholic steatohepatitis, or NASH, which is characterized by liver inflammation and can progress to advanced scarring (cirrhosis) and liver failure.
We aimed to understand the basis of this fibrotic scarring and identify drug targets that could lead to new treatments for advanced NASH by studying hepatic stellate cells, which are the key scar-producing cells in the liver.
Scott L. Friedman
NASH is also the leading cause of liver cancer in the world. Because advanced stages of NASH are caused by the accumulation of fibrosis or scarring, attempts to block fibrosis are at the heart of NASH treatment efforts, but no drugs are currently approved for this purpose, according to the researchers.
The researchers used single-nuclear sequencing in parallel studies of both mouse models of NASH and human liver tissue from nine NASH patients and two controls. They discovered a total of 68 pairs of potential drug targets shared by the two species. Furthermore, as a proof of concept, the researchers pursued one of these pairs by testing an existing cancer drug in mice.
“We aimed to understand the basis of this fibrotic scarring and identify drug targets that could lead to new treatments for advanced NASH by studying hepatic stellate cells, which are the key scar-producing cells in the liver,” said senior study author Scott L. Friedman, MD, Irene and Dr. Arthur M. Fishberg Professor of Medicine, Dean for Therapeutic Discovery, and Chief of Liver Diseases at Icahn Mount Sinai.
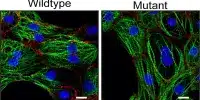
“By combining this new glass liver imaging approach – an advanced tissue clearing method that allows for deep insight – with gene expression analysis in individual stellate cells, we have revealed an entirely new understanding of how these cells generate scarring as NASH progresses to late stages.”
The researchers discovered that in advanced disease, stellate cells form a dense network, or meshwork, of interactions among themselves, facilitating the 68 previously unknown interaction pairs.
“We confirmed the importance of one such pair of proteins, NTF3-NTRK3, using a molecule that had already been developed to block NTRK3 in human cancers and repurposed it to establish its potential as a new drug to fight NASH fibrosis,” said first author Shuang (Sammi) Wang, Ph.D., an instructor in the Division of Liver Diseases. “This new understanding of fibrosis development suggests that advanced fibrosis may have a unique repertoire of signals that accelerate scarring, which represents a previously unrecognized set of drug targets.”
The researchers hypothesize that as the disease progresses, the circuitry of how cells communicate with one another evolves, so some drugs may be more effective earlier and others later. Furthermore, the same drug may not be effective at all stages of disease.
The researchers are currently collaborating with Icahn Mount Sinai chemists to improve NTRK3 inhibitors for the treatment of liver fibrosis. Following that, the researchers intend to functionally screen all candidate interactors in a cell-culture system before testing them in preclinical models of liver disease, as they did with NTRK3. They also hope to expand their research to see if similar interactions between fibrogenic cells cause fibrosis in other tissues such as the heart, lungs, and kidneys.
“An autocrine signalling circuit in hepatic stellate cells underpins advanced fibrosis in non-alcoholic steatohepatitis,” according to the paper’s title.