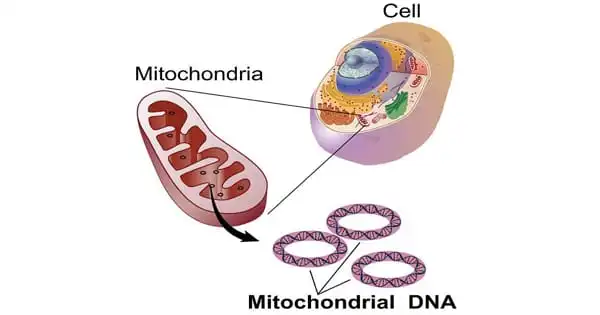
Mitochondrial illness, also known as mitochondrial dysfunction, refers to a range of conditions that affect the mitochondria, which are small compartments found in practically every cell of the body. The primary purpose of the mitochondria is to generate energy. More mitochondria are required to produce more energy, particularly in high-energy-demanding organs such as the heart, muscles, and brain. When the amount or function of mitochondria in a cell is disrupted, less energy is produced, and organ failure occurs.
Researchers demonstrated that the benefits of endurance exercise can vary depending on the type of mutation involved in mitochondrial disease, and while the benefits of exercise tend to outweigh the risks, the mitochondrial genetic status of patients should be taken into account when recommending exercise as therapy.
Mitochondria are the primary source of energy production in our cells, and endurance exercise has been shown to boost mitochondrial function. However, the benefits of exercise in patients with primary mitochondrial disorders remained mainly unknown. These diseases are varied and caused by a variety of genetic abnormalities.
There was little agreement among doctors who see patients with mitochondrial illness on whether endurance exercise genuinely gives advantages. Exercise promotes the formation of more mitochondria, but if those mitochondria still have the abnormalities associated with primary mitochondrial illness, exercise may put some patients at danger.
Patrick Schaefer
Researchers at Children’s Hospital of Philadelphia (CHOP) demonstrated in a new study that the benefits of endurance exercise can vary depending on the type of mutation involved in mitochondrial disease, and that while the benefits of exercise outweigh the risks, patients’ mitochondrial genetic status should be considered when recommending exercise as therapy. The findings were published today in the Proceedings of the National Academy of Sciences online.
Primary mitochondrial illnesses are the most common hereditary metabolic abnormalities, affecting one out of every 4,200 persons. Hundreds of different mutations in the nuclear DNA (DNA within our cells) or mitochondrial DNA can cause these illnesses (mtDNA, or the DNA within the mitochondria within our cells). There are few universal treatments for these people. Endurance exercise, on the other hand, has been demonstrated to improve mitochondrial function in healthy people and lower the chance of acquiring secondary metabolic problems such as diabetes or neurodegenerative diseases.
These suggestions, however, were based on healthy adults who did not have primary mitochondrial dysfunction. As a result, researchers wanted to investigate whether endurance training is useful for these patients and whether they gain from it.
“There was little agreement among doctors who see patients with mitochondrial illness on whether endurance exercise genuinely gives advantages,” said Patrick Schaefer, Ph.D., a postdoctoral fellow at CHOP’s Center for Mitochondrial and Epigenomic Medicine and the study’s first author. “Exercise promotes the formation of more mitochondria, but if those mitochondria still have the abnormalities associated with primary mitochondrial illness, exercise may put some patients at danger.”
Because of the heterogeneity of primary mitochondrial disease among patients, the researchers used animal models to study five mutations responsible for the disease. The goal of the study was to determine the relationship between mitochondrial mutations, endurance exercise response, and the underlying molecular pathways in these models with distinct mitochondrial mutations.
The study discovered that depending on the mutation, endurance exercise had varying effects on the models. Exercise improved responsiveness in the complex I model with the mtDNA ND6 mutation. The model with a CO1 mutation affecting complex IV had much fewer favorable effects associated with exercise, but the model with an ND5 complex 1 mutation did not respond to exercise at all. In the model lacking nuclear DNA Ant1, endurance exercise exacerbated cardiomyopathy.
Furthermore, the researchers were able to correlate the gene expression profile of skeletal muscle and heart in the model with exercise response and identified oxidative phosphorylation, amino acid metabolism, and cell cycle regulation as key pathways in exercise response, suggesting how the model could be adapted to study exercise responses in humans with primary mitochondrial disease.
Despite the models’ inconsistent answers in this study, the authors conclude that the benefits of exercise outweigh the hazards in most circumstances. However, when proposing therapeutic workouts, the patient’s physical and mitochondrial health should be considered. Furthermore, the work could aid researchers in identifying biomarkers and pathways that can be used to predict the mitochondrial response to exercise in both mitochondrial patients and the general population with diverse mitochondrial haplogroups.
“This work is fundamental in demonstrating that people with different mitochondrial bioenergetics will respond differently to endurance exercise,” said senior study author Douglas C. Wallace, Ph.D., director of CHOP’s Center for Mitochondrial and Epigenomic Medicine and the Michael and Charles Barnett Endowed Chair in Pediatric Mitochondrial Medicine and Metabolic Diseases. “This has significant implications for anyone ranging from athletes to patients with mitochondrial illness and everyone in between.”