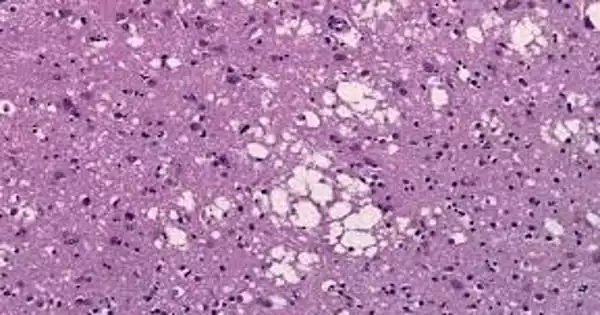
Prion diseases, such as Creutzfeldt-Jakob disease (CJD), are fatal dementia syndromes caused by the formation of prion protein aggregates. The mechanism by which these aggregates form within and kill brain cells has never been fully understood, but a new study from Scripps Research suggests that the aggregates kill neurons by damaging their axons, the narrow nerve fibers through which they send signals to other neurons.
Protein aggregates in axons, as well as axonal swellings and other signs of dysfunction, are also early features of other neurodegenerative disorders, such as Alzheimer’s and Parkinson’s. The discovery of how these prion aggregates form in axons and how to inhibit them, reported in Science Advances, may have far-reaching implications beyond prion diseases.
“We hope that these findings will lead to a better understanding of prion and other neurodegenerative diseases, as well as new treatment strategies,” says study senior author Sandra Encalada, PhD, Arlene and Arnold Goldstein Associate Professor in the Department of Molecular Medicine at Scripps Research.
A complex of key proteins was also identified as being responsible for steering PrP into axons and causing aggregation associated with large axonal swellings. We hope that these findings will lead to a better understanding of prion and other neurodegenerative diseases, as well as new treatment strategies.
Sandra Encalada
The researchers observed mutant, disease-causing copies of the prion-disease protein PrP forming large aggregates in neurons’ axons but not in the neurons’ main cell bodies in their study. Following the formation of these aggregates, there were signs of axon dysfunction and, eventually, neuronal death. The researchers discovered evidence that neurons’ waste-disposal processes are normally capable of dealing with such aggregates when they accumulate within or close to neurons’ main cell bodies, but are far less capable when the aggregates accumulate far out within axons.
A complex of key proteins was also identified as being responsible for steering PrP into axons and causing aggregation associated with large axonal swellings, according to the researchers. They demonstrated that by silencing any of these proteins, they could prevent the formation of aggregates and protect neurons from damage and death.
Vulnerable axons
CJD is the most common human prion disease, with approximately one case per million people worldwide each year. The majority of cases are thought to arise spontaneously when PrP is altered in the brain and begins to aggregate. Because these aggregates form through a chain reaction that draws in healthy copies of PrP, they can transmit CJD from one person to another in rare cases, such as during corneal transplant surgery. About 15% of cases are hereditary, caused by mutations that increase the likelihood of PrP aggregation. Prion disorders occur in other mammals and are thought to be caused by toxic aggregations of PrP proteins from different species.
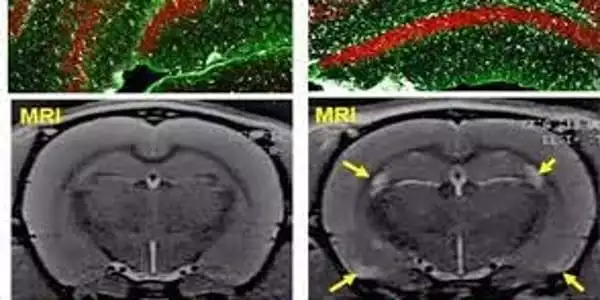
Encalada’s team studied the initial accumulation of PrP aggregates in axons using mouse brain cells containing mutant PrP and microscopic motion-picture techniques. The axon of a neuron is frequently very long in relation to its main body, the soma, and has been found to be uniquely vulnerable to disruptions of its delicate systems for transporting essential molecules and disposing of waste.
PrP’s normal function in neurons has never been determined, but the protein appears to be normally secreted from the soma and axon via sac-like containers called vesicles, where it sometimes returns to be recycled or degraded as waste. In their experiments, the researchers discovered that mutant PrP produced in the soma is also largely encapsulated in vesicles that travel into the axon along railways known as microtubules.
This movement is facilitated by a somewhat complex vesicle trafficking system, which shunts much of the PrP far out into axons, where PrP-containing vesicles gather and merge. In this situation, mutant PrP forms large aggregates – Encalada refers to them as endoggresomes – that axons can’t get rid of. When compared to neurons with normal PrP, the aggregates cause axonal swellings and other signs of dysfunction, such as reduced neuronal calcium signaling, and ultimately a much faster neuronal death rate.
The researchers also discovered a method to prevent the formation of endoggresomes. They discovered four proteins, Arl8, kinesin-1, Vps41, and SKIP, that are in charge of directing PrP-containing vesicles into axons, transporting them to the soma, and merging them with other PrP-containing vesicles to cause aggregate formation. When they silenced any of these proteins, far fewer PrP-containing vesicles entered axons, the axons exhibited few or no signs of aggregation, and the neurons functioned normally or nearly normally, surviving just as well as normal brain cells.
The findings raise the tantalizing prospect that prion diseases, and possibly many other protein-aggregate diseases of the brain, can be prevented or treated by temporarily disrupting the trafficking process that transports vesicle-encapsulated, aggregate-prone proteins out into axons.
“We’re very excited about finding molecules that can inhibit this aggregate-forming pathway and studying the effects of such inhibitors in prion and other neurodegenerative disease animal models,” Encalada says.