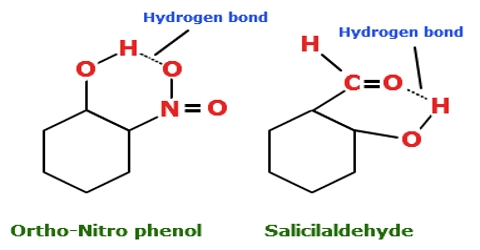
Intramolecular hydrogen bonding (i.e. hydrogen bonding between the hydrogen atom in one part of the molecule with an electronegative atom like oxygen or nitrogen in another part of-the same molecule) can also take place. Intramolecular hydrogen bonds are those which occur within one single molecule. For example, in O- nitrophenol the hydrogen atom of the O — H group forms a hydrogen bond with one O-atom of the NO2 group of the same molecule as shown below:
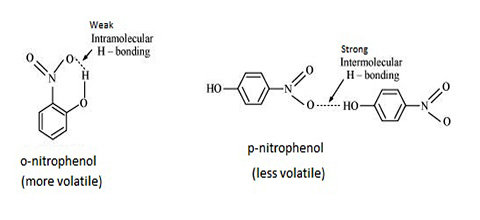
- Figure (a): T.N.R. Intramolecular hydrogen bonding in o-nitrophenol
- Figure (b): T.N.R. Intramolecular hydrogen bonding in p-nitrophenol
Intramolecular hydrogen bonding in o-nitrophenol causes this compound to have a lower boiling point than p-nitrophenol where intermolecular hydrogen bonding (hydrogen bonding between two or more molecules) is present. In p-nitrophenol intramolecular hydrogen bonding is not possible became of the distance between the atoms which form such a bond. This is shown in Figure (b), o-nitrophenol is much less soluble in water compared to p-nitrophenol. This is because p-nitrophenol can form hydrogen bonds with water whereas o- nitrophenol cannot.