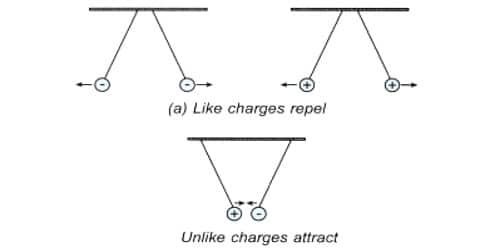
Like Charges, Repel and Unlike Charges Attract Each Other: Experimental Verification
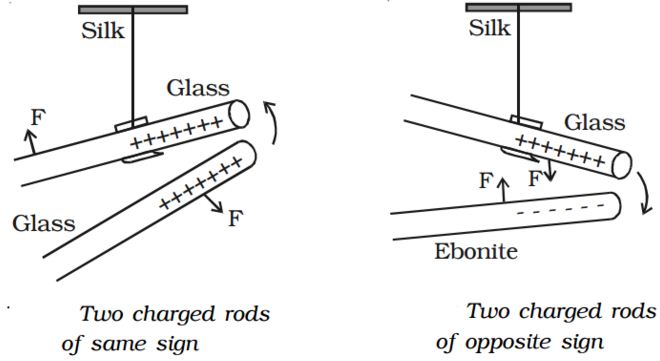
A charged glass rod is suspended by a silk thread, such that it swings horizontally. Now another charged glass rod is brought near the end of the suspended glass rod. It is found that the ends of the two rods repel each other (Fig 1). However, if a charged ebonite rod is brought near the end of the suspended rod, the two rods attract each other (Fig 2). The above experiment shows that as charges repel and unlike charges attract each other.
The property of attraction and repulsion between charged bodies have many applications such as electrostatic paint spraying. powder coating. fly-ash collection in chimneys. ink-jet printing and photostat copying (Xerox) etc.
Summary:
(i) If a glass rod is rubbed with a silk cloth, it acquires a positive charge while the silk cloth acquires an equal amount of negative charge.
(ii) If an ebonite rod is rubbed with fur, it becomes negatively charged, while the fur acquires equal amount of positive charge. This classification of positive and negative charges was termed by the American scientist, Benjamin Franklin.
Thus, charging a rod by rubbing does not create electricity, but simply transfers or redistributes the charges in a material.
Theoretical Explanation –
There are two fundamental principles of charge interactions are used throughout to explain the immense assortment of static electricity phenomena. These two types of electrical charges – positive and negative – are said to be contradictory types of charge. And we know from the fundamental principle of charge contact, a positively charged entity will attract a negatively charged object. Since the electron is much lesser and lighter than a proton, when they are concerned with each other due to their different charges, the electron generally does most of the moving. Oppositely charged objects will apply an attractive persuade upon each other. In dissimilarity to the attractive force between two objects with contradictory charges, two objects that are of like charge will repel each other. This is because the protons have more accumulation and are harder to get moving. Although electrons are very diminutive their negative electrical charges are tranquil rather strong. That is, a positively charged object will exert a repulsive force upon a second positively charged object. Remember, the negative charge of an electron is the same as the positive electrical charge of the much larger in size proton. This way the atom stays electrically balanced. Similarly, a negatively charged object will exert a repulsive force upon a second negatively charged object. Objects with like charge repel each other.
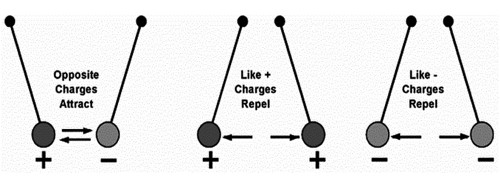
So, finally, we can say that,
Objects can be positively charged, negatively charged or neutral (no charge). A material that gains electrons becomes negatively charged, while a material that loses electrons becomes positively charged.
When a charged object comes near to another object they will either attract or repel each other. Two electrons will tend to repel each other because both have a negative electrical charge. Two protons will also tend to repel each other because they both have a positive charge. On the other hand, electrons and protons will be concerned with each other because of their dissimilar charges.
If the charges are similar – they repel. If the charges are opposite – they attract. If one is charged and the other is not – they attract.