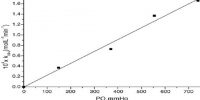
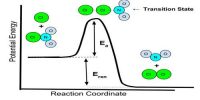
Luminescence
As a result of the absorption of light; molecules or atoms are excited, i.e. have more energy. In nearly all cases some or all of the extra energy is dissipated as heat. When the substance does not undergo photo-chemical reaction, however, some of the energy is re-emitted as radiation. The substance is then said to show luminescence. Luminous simply means giving off light; most things in our world produce light because they have energy that originally came from the Sun, which is the biggest, most luminous thing we can see. The emission of light in the visible region of the spectrum is attributed to the return of one or more of the outer electrons of the excited molecules or atoms to the normal position as a result of collisions with other atoms or molecules. De-activation of the excited molecules may also occur by chemical, electrical or other forms of interaction.
Luminescence may be seen in neon and fluorescent lamps; television, radar, and X-ray fluoroscope screens; natural materials such as luminol or the luciferins in fireflies and glowworms; definite pigments used in outdoor advertising; and also usual electrical phenomena such as lightning and the aurora borealis.
Examples of Luminescence
Chemoluminescence – This process creates light through a chemical reaction. Common applications are glow sticks (cyalume sticks) and the forensic application for locating blood using luminol.
Crystalloluminescence – Light is made during crystallization and it is theorized that the light comes from small fractures in the crystal.
Electroluminescence – This occurs when an electromagnetic field is applied to solid or gaseous material. This excites the molecules and the substance glows. LEDs, or light-emitting diodes, are found in flat panel televisions and computer monitors.