Mean Free Path
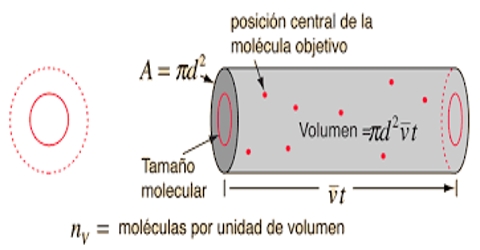
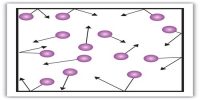
The mean free path is the distance that a molecule travels between collisions. Mean free path of a gas molecule is defined as “the average distance a molecule travels between two successive collisions”. The actual distance travelled by a molecule between consecutive collisions may vary considerably from time to time. Sometimes it may move a long distance before hitting another molecule, at other times two consecutive collisions may take place within a very short distance. It may be shown that the mean free path, l, of a gas is given by;
l = [(1/√2) x 1/(πσ2n)]
Where n is the number of molecules in unit volume and σ is known as the collision diameter, i.e., the distance between centers of molecules the time of their closest approach. This value is slightly larger than the molecular diameter as shown in Figure.
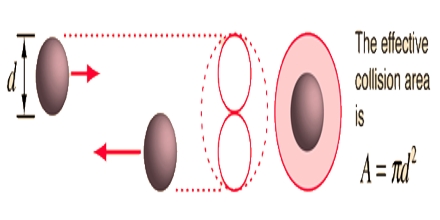
During collision the molecules do not actually touch each other because of mutual repulsion when they come very close to each other. Since σ is not very much different for ordinary gases the mean free path of a gas depends mainly on n.