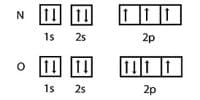
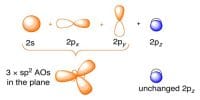
The amount of energy required to remove the most loosely held electron from a neutral gaseous atom of an element in its ground state to form positive ion is called ionization potential of that element.
It is dependent upon several factors:
(i) Atomic size
(ii) Increase of positive charge at nucleus
(iii) Principal quantum number
(iv) Atomic number / electronic configuration
(v) Shielding effect.