Mount Sinai’s Icahn School of Medicine researchers have developed a better grasp of the varied effects of JAK inhibitors, also known as modulators, in inflammation across distinct cell types and tissues. Their findings indicate that a more precise strategy is needed to potentially broaden JAK inhibitor use to a broader range of allergy and inflammatory conditions. Details on the discoveries were published in the issue of the journal Cell.
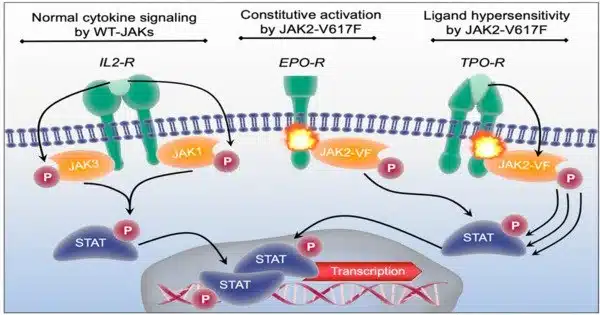
JAK1 is an important protein in the body that facilitates cell communication and regulates the immune system. It is one of a group of proteins that transfer signals from a cell’s surface to its inner. Controlling JAK1 activity is also vital in treating rheumatoid arthritis and some malignancies.
Current JAK inhibitors are effective against inflammation in disorders such as eczema, but the study offers a more nuanced strategy to controlling JAK activity in ailments such as asthma. The researchers believe that increasing, rather than blocking, JAK activity in lung neurons could be a transformative method, as opposed to standard JAK inhibitors, which primarily target immune cells.
This may explain why JAK1-selective inhibitors, while highly successful in atopic dermatitis, have not advanced for asthma treatment and implies that JAK1 signaling has varying or even opposing effects in different cell types and tissues.
Brian Kim
A mouse engineered with a patient-specific mutation in the JAK1 gene was used in the study to show how this mutant protein causes disease and how it could possibly be used for broader therapeutic purposes. The study found that active JAK1 signaling has tissue-specific consequences, including an unexpected immunoregulatory role in lung sensory neurons, which lowers lung inflammation.
“This may explain why JAK1-selective inhibitors, while highly successful in atopic dermatitis, have not advanced for asthma treatment and implies that JAK1 signaling has varying or even opposing effects in different cell types and tissues,” says lead study author Brian Kim, MD, MTR, FAAD, the Sol and Clara Kest Professor of Dermatology, Vice Chair for Research, and Director of the Mark Lebwohl Center for Neuroinflammation and Sensation at Icahn Mount Sinai.
The study involved a type of JAK1 gain-of-function (GOF) mutation, first reported by study co-author Stuart Turvey, MBBS, DPHIL, FRCPC in 2017, from patients with an immune dysregulatory and hypereosinophilic syndrome characterized by severe eczema and asthma. A JAK1 GOF mutation is a change in the gene that encodes the JAK1 protein, making it more active than normal. This increased activity can lead to overactive immune responses and may cause health problems like autoimmune diseases or cancer.
“As a pediatrician, I started this work with a commitment to finding a diagnosis and treatment for a family where three members had severe eczema, asthma, and other allergic manifestations. It turned out that they were the first people in the world recognized to have a genetic change causing gain of function of JAK1,” says Dr. Turvey, Professor, Division of Immunology, Department of Pediatrics, Faculty of Medicine, and Canada Research Chair in Pediatric Precision Health, BC Children’s Hospital and The University of British Columbia, Vancouver, Canada. “Today’s publication, led by Dr. Kim and his team at Mount Sinai, is a powerful example of globally collaborative translational research where they started with this single family and have now generated fundamental insights into the interactions between the human immune system and the nervous system. This is precision medicine in practice.”
Previous research by Dr. Kim and his team revealed that JAK1 signaling, typically responsible for regulating immune cells’ inflammatory response, is also present in neurons and controls the sensation of itch. In their new study, Drs. Kim and Turvey created mice with the same genetic mutation as the patients to better understand why JAK1 inhibitors work for some allergy and inflammatory conditions.
In the lung neurons of the mice, the JAK1 mutant protein reduced inflammation caused by exposure to mold by producing substances that suppress inflammation. However, the mice still showed the same skin condition as the original patients, indicating that JAK1 signaling has different effects in different cells and even within the same cell type in different parts of the body.
“Better understanding of JAK signaling in different parts of the body not only helps us discover new things about biology but also gives us a glimpse into how JAK medicines might be used in the evolving landscape of innovative treatments,” Dr. Kim said.
The researchers will then look into how other genes in the JAK pathway may influence tissue-specific disease patterns. “For example, another protein named STAT6 is located downstream of the JAK1 pathway. Dr. Turvey discovered patients who had mutations in these genes and develop similar allergy symptoms. However, whether these individual mutations might inform therapeutic options for certain diseases is still a really intriguing area of research,” adds Dr Kim.