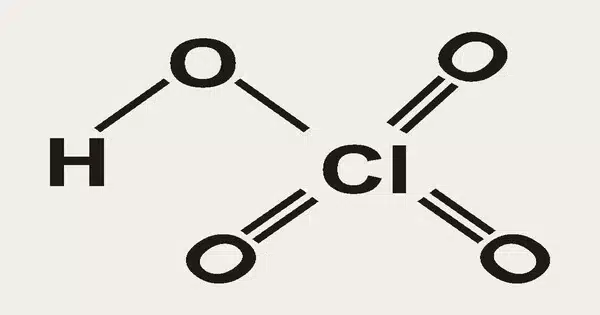
Perchloric acid has the formula HClO4 and is a mineral acid. It is a strong mineral acid with strong oxidizing properties. This colorless compound, which is usually found as an aqueous solution, is a stronger acid than sulfuric acid, nitric acid, and hydrochloric acid. It is a strong oxidizer when heated, but aqueous solutions containing up to 70% by weight at room temperature are generally safe, exhibiting only strong acid features and no oxidizing properties.
Perchloric acid is useful for preparing perchlorate salts, particularly ammonium perchlorate, an important component of rocket fuel. It is one of the most powerful acids found in laboratories and various industrial applications. It is extremely corrosive and easily reacts with other chemicals to form potentially explosive mixtures.
Properties
Perchloric acid is a colorless and odorless liquid at room temperature. It is highly corrosive to metals, so it is usually stored in glass containers or plastic bottles specifically designed to handle its reactivity. It is a very stable acid and does not readily decompose, making it suitable for various laboratory purposes.
- Chemical formula: HClO4
- Molar mass: 100.46 g/mol
- Appearance: colorless liquid
- Odor: odorless
- Density: 1.768 g/cm3
- Melting point: −17 °C (1 °F; 256 K) (72% aqueous solution); −112 °C (anhydrous)
- Boiling point: 203 °C (397 °F; 476 K) (azeotrope)
- Solubility in water: Miscible
- Acidity (pKa): −15.2 (±2.0); ≈ −10
- Conjugate base: Perchlorate
Production
Perchloric acid is produced industrially by two routes. The traditional method exploits the high aqueous solubility of sodium perchlorate (209 g/100 mL of water at room temperature). Treatment of such solutions with hydrochloric acid gives perchloric acid, precipitating solid sodium chloride:
NaClO4 + HCl → NaCl + HClO4
The concentrated acid can be purified by distillation. The alternative route, which is more direct and avoids salts, entails anodic oxidation of aqueous chlorine at a platinum electrode.
Laboratory preparations
When barium perchlorate is treated with sulfuric acid, barium sulfate precipitates, leaving perchloric acid. It can also be made by boiling nitric acid and ammonium perchlorate while adding hydrochloric acid. Because of a concurrent reaction involving the ammonium ion, the reaction produces nitrous oxide and perchloric acid, which can be concentrated and purified significantly by boiling off the remaining nitric and hydrochloric acids.
Uses
Perchloric acid is primarily produced as a byproduct of the production of ammonium perchlorate, which is used in rocket fuel. The increased production of perchloric acid has resulted from the growth of rocketry. Every year, several million kilograms are produced. Perchloric acid is a well-proven material for etching liquid crystal displays, critical electronics applications, and ore extraction, and it has unique properties in analytical chemistry. It is also an important component in the etching of chrome.
Safety Precautions
Because of its high reactivity and potential for violent reactions, handling perchloric acid requires extreme caution. To avoid the accumulation of potentially explosive perchlorate salts, it should be used in a well-ventilated fume hood. To reduce the risks associated with its use, special equipment and storage guidelines are required.
Perchloric acid is extremely corrosive to tissues and can cause severe burns upon contact. Its vapors can cause respiratory irritation if inhaled. When working with this acid, use proper personal protective equipment (PPE).