Since heat flows spontateneously from object with high T to an object with low T, the temperature of a body changes when the heat is added or removed. The heat Q is proportional to the size of the object [mass m ] and also depends on the type of material,
Q = mc ΔT
where c is the specific heat Calorimetry. Since heat is a form of energy [just like kinetic and potential energy], the unit of the specific heat is c = J/(kg °C). Heat must be added or removed during a phase change [gas-liquid (evporation or condensation), liquid-solid (melting or freezing), or gas-solid (sublimation)]. Because the temperature is constant during a phase change,
Q = mL,
where the latent heat L has the unit [LI = J/kg. The specific and latent heat for materials is measured experimentally. The specific heats are different for the different phases of the same substance and likewise, the latent heats are different for different phase transformations. Furthermore, specific and latent heats are different for different substances.
Example: The best way to make hot chocolate is by putting steam into chilled milk. An Espresso machine produces vapor at 100°C and pressure P = 9.0 x 105 Pa [or 9 atm]. The barista puts msteam = 50.4 g [ V = 9.6 x 10-3 m3 or 9.6 L] of steam into the chilled milk. A 12-ounce cup of milk contains 0.36 kg of milk at a temperature of 6°C. Find the temperature of the Hot Chocolate! Ignore the specific heat of the cup. Useful data: Specific heat of water Cwater = 4, 186 J/(kg •° C), Specific heat of milk Cmilk = 3, 890 J/(kg •° C) , and latent heat of vaporization of water Lwater = 22.6 x 105 J/kg.
Solution:We have for the heat given off by the vapor [and hot water] and the absorbed heat:
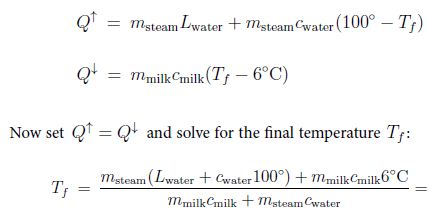
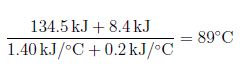
That’s about 1900 F.












