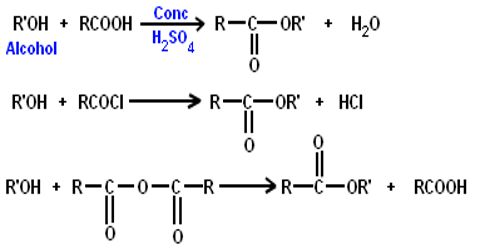
Preparation of Esters:
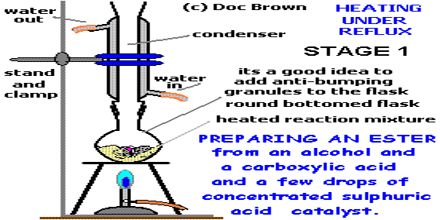
From acid by Esterification: If carboxylic acid & alcohol are heated with conc. H2SO4 or HC1(g), ester & water are produced. The reaction is reversible. In this reaction, H2SO4 or HCl are used as the dehydrating agent. The -OH group of carboxylic acid takes part in water formation, not the -OH of alcohol. Esterification is the name given to a reaction in which a Carboxylic acid combines with an alcohol in the presence of concentrated sulphuric acid to form an ester. For example, Ethanoic acid reacts with carbonates and hydrogencarbonates to give rise to a salt, carbon dioxide and water.
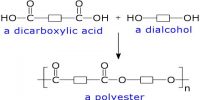
Esters are derivative of carboxylic acids in which the -OH group has been replaced by -OR group, where R may be an alkyl/aryl group. It is an organic compound where the hydrogen in the compound’s carboxyl group is replaced with a hydrocarbon group.
This method can be used for converting alcohols into esters, but it doesn’t work with phenols – compounds where the -OH group is attached directly to a benzene ring. Phenols react with carboxylic acids so slowly that the reaction is unusable for preparation purposes.