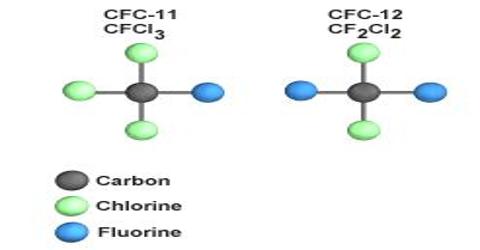
The chloro-flouro derivatives of methane & ethane are known as “C.F.C”. They are commercially known as “Freons”. Chlorofluorocarbon (CFC) is an organic compound that contains carbon, chlorine, and fluorine, produced as a volatile derivative of methane and ethane. Among the CFCs. Freon-11 (CCl3F); Freon -12 (CC12F2) & Freon -21 ( F2ClCC.ClF2) are important.
The chemical reaction for CFC is: CCl3F = CCl2F + Cl.
Properties:
- CFCs are colorless, odorless, non toxic inflammable gaseous substance.
- They can easily be convened into liquids by applying little pressure.
- They have low boiling points, the B P of Freon -11 & Freon -12 is 240C & 300C respectively.
- They are insoluble in water.
Physical Properties of chloro-flouro carbon:
- Molecular Weight: 187.38 (Freon-113)
- Boiling Point: 47.70C
- Melting Point: -36.40C
- Water Solubility: 170 mg/l at 250C
Uses:
- Mostly used as refrigerant in air-conditioner, refrigerator etc.
- Production of aerosol, propellant gas, hair spray, perfume, plastic foam etc.
- As CFCs cause harm to the Ozone layer of atmosphere, so now a day’s their uses become very limited.
- CFC gases are relatively stable compounds used as refrigerant gases in air conditioning units, freezers, refrigerators and other household appliances.