A reversible reaction is a chemical reaction that results in an equilibrium mixture of reactants and products. If steam is passed over the hot iron, hydrogen and tri-iron tetroxide are produced as shown below:
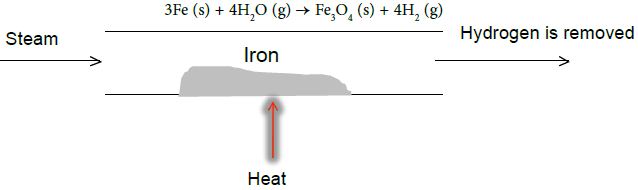
This reversible reaction can be reversed by passing hydrogen over tri-iron tetroxide to produce iron and steam as shown below
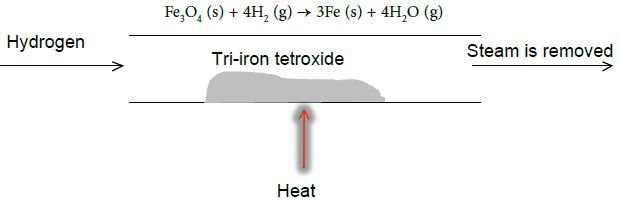
Under the conditions shown each reaction is one way with the products being removed from the reaction mixture.
Under these conditions, no reverse reaction can happen. To allow the reverse reaction to happen products must not be lost from the system i.e. the reaction must be carried out in a closed system.
Reactions in a closed system: If the iron were to be heated in a closed system (where no reactants may be added and no products lost), then at some point the composition of the reaction mixture would remain constant, and all four species would be present as shown in the following equilibrium.
3Fe (s) + 4H2O (g) ≈ Fe3 O4 (s) + 4H2 (g)