Rutherford’s Atom Model
In 1911, famous scientist Rutherford proposed a model of an atom. Through extensive experiments he observed the deflection or scattering from different heavy metals of α- particles emitted from radioactive substances and proposed this structural model of the atom. In honor of his name, this model is called Rutherford’s atom model.
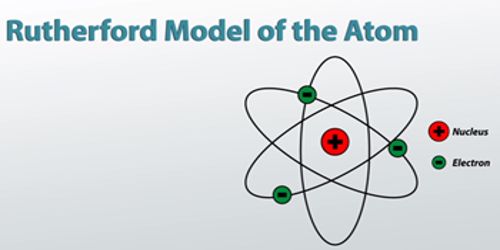
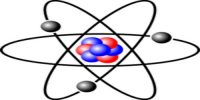
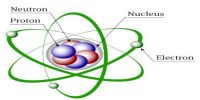
According to this model, all positive charges of the atom are considered to be concentrated in a small region at the center. The concentrated mass of the charged center is called the nucleus. The nucleus is the source of energy of the atom. Its radius is about 10-14 m. The radius of the atom is about 10-10 m. Except for the nucleus, the rest space inside the atom is almost empty. In this empty portion, some fixed number of electrons revolves around the nucleus in some circular orbits.
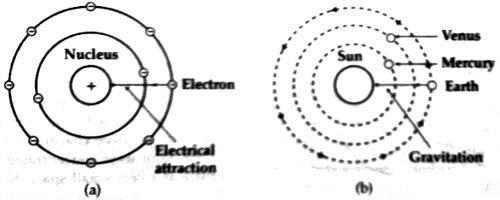
Since the centrifugal force originated due to the rotation of electrons and the Coulomb’s force between the nucleus and the electrons are equal and opposite, hence the electrons revolve around the nucleus in different stable orbits [Fig. (a)]. Rutherford told that this model can be compared with the solar system [Fig. (b)]. As the planets rotate around the sun, similarly electrons rotate around the nucleus.
The Coulomb’s attractive force between the positively charged nucleus and negatively charged electron rotating around the nucleus acts in this case as a centripetal force.
Since there is a similarity between the solar system with that of Rutherford’s hypothetical model, hence this model is called the planetary model.