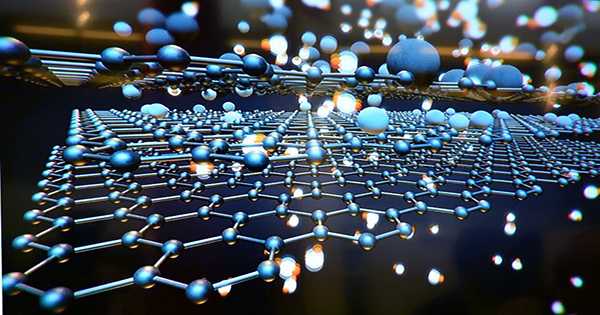
Carbon is a pretty amazing material and pure carbon has a variety of uses and properties. Carbon is surprisingly diverse in our pencils, from the incredible hardness of diamonds to the versatility of graphite. The two-dimensional structures are incredible and contain things like graphene, carbon nanotubes, and fullerenes that exhibit a variety of useful properties. Now a new 2D carbon structure has been discovered. It is called schwarzite and it has been predicted and modeled for decades but now only researchers have been able to create it.
The new structure was created by scientists in South Korea and Japan with a special type of silicon dioxide crystal (sand), known as zeolite, filled with carbon. Manufacturers were investigating whether these carbon-filled zeolites would have attractive properties. An international team led by researchers at the University of California, Berkeley, has proven that the three structures that the Japanese-Korean team created are in fact many years of sought-after squirrels. Their findings have been published in the Proceedings of the National Academy of Sciences.
An important property of schwarzite is its curvature. Schwartz is the first carbon structure with negative curvature and somewhat shaped like wood. Schwartz has the smallest area for configuration.
The team created a computer simulation of zeolite crystals and modeled how carbon atoms can be arranged. Studies have shown that to date 200 zeolites have been created; only 15 square zeolites can be used as a template to create. However, researchers believe that there are millions of possible ways to make zeolite, so there may be many more ways to make schwarzite.
Lead author Efrem Braun, from UC Berkeley, said in a statement, “These negatively-curved carbons were very difficult to synthesize on their own, but it has been proven that you can catalyze a carbon film on the surface of a zeolite.” “However, to date synthesized have been created by selecting zeolite templates through trial and error. We reasonably provide very simple instructions for creating schwarzites and we have shown that by choosing the right zeolite you can tune the schwarzites to optimize the properties you want.”
Schwarzites are particularly suited to storing a large electric charge, making them efficient capacitors. They can be made in such a way that they can store other molecules and even act as catalysts. Similarly for graphene, finding an efficient, high-quality, and expensive way to produce the structure will be the key to its commercialization.